1,2 & 3- Periodicity, Group 2 and Group 7
1/78
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
79 Terms
How can we use calcium carbonate and oxide to remove sulfur dioxide gas emissions?
When we burn fossil fuels, harming sulfur dioxide (a pollutant) is produced.
Wet scrubbing is a process where an alkali can neutralise sulfur dioxide in flue gases
Wet scrubbing involves dissolving calcium carbonate/oxide in water and spraying it on acidic sulphur dioxide gas
The product is calcium sulfite which can be used in production of plasterboard.
What do all elements in the same period have in common?
Same number of shells
What do all elements in the same group have in common?
Same number of electrons in their outer shell
Explain the trend in atomic radius in the periodic table
Along a period - atomic radius decreases due to increased nuclear charge. The outer electrons are pulled in closer to the nucleus due to greater forces of attraction
Down a group - atomic radius increases due to increased number of shells, there is a larger distance from the outer shell and the nucleus so there is a weaker attraction. This outweighs the increased proton number.
Explain the trend of ionisation energies in the periodic table
Along a period - ionisation energy increases. Decreasing atomic radius and increasing nuclear charge results in outer electrons being held more strongly so more energy is required to remove the electrons.
Down a group - ionisation energy decreases. The increased amount of shielding means less energy is required to remove the outer electron as it is not held so strongly.
Who was Mendeleev?
Chemist who organised the periodic table in order of increasing atomic weight, left gaps for undiscovered elements.
Placement of hydrogen and helium in the periodic table
Hydrogen - placed above group 1 as it usually forms +1 ions but otherwise is not similar due to hydrogen being a gas and group 1 elements being reactive metals.
Helium - placed above noble gases because of its properties but not a p block element
Why does the melting point increase from sodium to aluminium?
Due to the strength of the metallic bonding.
As you go from left to right, the positive charge on the ions increases. More electrons are delocalised so the attractive electrostatic force of attraction increases.
Using the graph, explain why the melting point of Phosphorus and Sulfur is lower than that of Silicon
Silicon is macromolecular meaning it has a very strong covalent structure. These covalent bonds require a lot of energy to overcome resulting in a high melting point.
Phosphorus and Sulfur are simple covalent molecules held together by weak van de waal forces. These intermolecular forces don't require as much energy to overcome hence Phosphorus and Sulfur have lower melting points
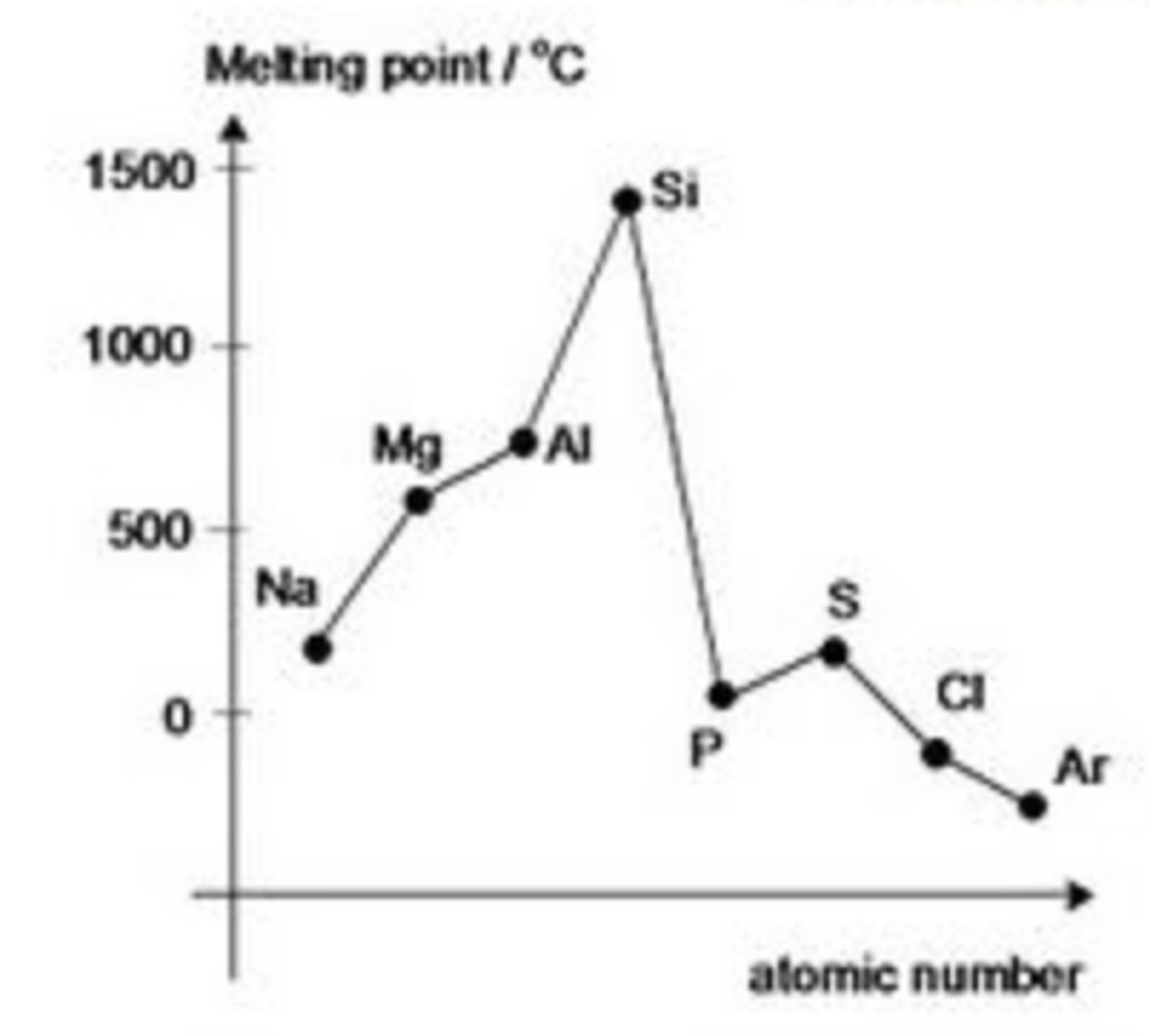
What do the melting points of metals depend on
-Bond strength
-Structure
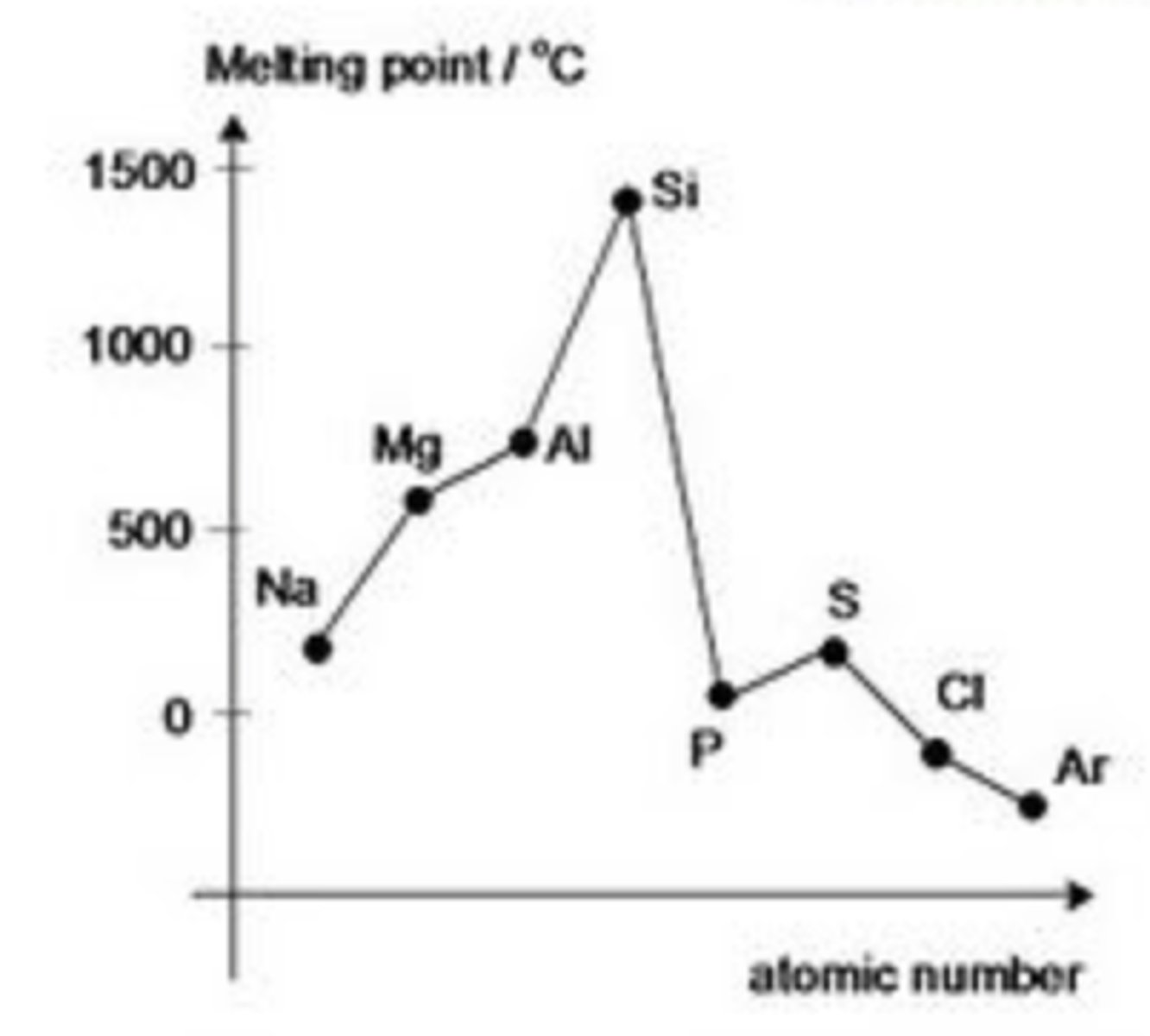
Why does the melting point decrease from phosphorus to chlorine in period 3? Explain argons melting point
Phosphorus (P4), Sulfur (S8) and Chlorine (Cl2) are simple molecular substances which consists of van de Waals.
The melting point depends on the strength of these van de Waals.
As sulfur is a larger molecule compared to phosphorus and chlorine, it increases slightly as the strength of the van de Waals are stronger
Argon - lowest melting point in period 3 as it exists as a monotonic element, weak van de Waals
Explain the trend in atomic radius down group 2 (alkali metals)
Atomic radius increases down a group due to there being more electrons and shielding
Explain the trend in melting point as we move down group 2
Melting points decrease down group 2 due to:
Size of the metal ions increase but the number of delocalised electrons stay the same. The charge on the ion (2+) also remains the same.
Larger ions have a greater distance between positive nuclei and delocalised electrons. This weakens the force, so it is easier to break the metallic bonds and hence less energy is required (lower melting point)
(magnesium is an exception due to structural differences)
Explain the trend in reactivity down group 2
Group two elements react with water to form bases
(form metal hydroxides, sulphates etc)
Reactivity increases down the group (Be unreactive) because the atoms get larger and shielding increases. The outer electron is further away from the nucleus hence it is easier to remove.
Magnesium reacts slowly with cold water but more vigorously with steam, this produced magnesium oxide instead of magnesium hydroxide
Explain the trend in solubility as we go down group 2 (reactions with water)
Sulphate solubility decreases down the group,
as a general rule if the negative ion (anion) has a double charge (SO42-) they become less soluble down the group.
Hydroxide solubility increases down the group,
as a general rule if the negative ion (anion) has a single charge (OH-) they become more soluble down the group.
BaSO4 is insoluble
Mg(OH) is sparingly soluble
Describe the tests for sulphates in a compound
1. Add HCL to remove any carbonates in the compound
(Barium carbonate is also a white precipitate, undistinguishable from barium sulfate and so would give a false positive if not removed). Acidified
2. Add barium chloride
A positive test results in a white precipitate (barium sulfate)
as the sulfate is insoluble in water.
For example, Ba2+ (aq) + SO42- (aq) -> BaSO2(s)
Solid (s) is the precipitate
Explain the use of neutralisation by alkali metals (group 2 elements)
-Neutralise acidic soils (Ca(0H)2)
- Antacids (Mg(OH)2 used to neutralise acidic stomach acid, reducing indigestion and heartburn
Ionic equation for neutralisation: H+(aq) + OH- (aq) --> H20(l)
Outline the use of barium meal in X-rays
-Patient drinks a suspension of barium sulfate, coating the lining of soft tissues such as the stomach
(the heavy barium atom is good at absorbing X-rays)
-Patient has X-ray, barium absorbs the X-rays (darkened images of X-ray)
-Helps to identify problems with the digestive track
Barium compounds are toxic, but insoluble and so cannot be absorbed into the blood and cause damage.
Extraction of titanium
Cannot reduce titanium oxide (TiO2) using carbon as you normally do to extract metals as the titanium would react with carbon to form titanium carbide (Tic) a brittle metal. Instead:
1. Titanium ore (TiO2) is converted into titanium chloride (TiCl4) by heating with carbon and chlorine gas.
2. TiCl4 passed through a fractional distillation column to increase purity.
3. Purified TiCl4 reduced using magnesium in a furnace
TiCl4 + 2Mg --> Ti + 2MgCl2
Commonly used in planes as its lightweight but strong
State the meaning of the term periodicity
Repeating pattern/trends of physical or chemical properties/reactions
Identify the element in period 4 with the largest atomic radius
Potassium as it has the smallest nuclear charge, but similar shielding/same number of shells
Equation for the reaction for magnesium with water and steam
Mg(s) + 2H20 (l) -> Mg(OH)2(aq) + H2 = mg + water
pH 9-10 as magnesium hydroxide is not very soluble in water, few hydroxide ions are produced
Mg reacts faster with steam to produce magnesium oxide as there is more energy
Mg(s) +H20(g) -> MgO(s) +H2
Why is sodium more reactive than magnesium?
It takes less energy to remove 1 electron (from Na) than it does to take 2 (from Mg) in a reaction.
More energy is required for magnesium to react
(Na group 1, Mg group 2)
How might we test the group 2 elements for their solubility? (completed in class)
Mixing solutions of group 2 salts with sodium hydroxide and recording the results.
Reaction of strontium (group 2) with water
Sr(s) + 2H20(l) -> Sr(OH)2aq + H2(g)
What do group 2 elements form when they react with water?
Metal hydroxide
(excluding magnesium which produces magnesium oxide when reacted with steam)
State an observation you would see when magnesium reacts with steam
-Bright light
-White powder
Explain why, in terms of structure and bonding, magnesium chloride has a high melting point (3)
Giant ionic lattice with lots of Mg2+ ions and Cl- ions/strong electrostatic forces of attraction/between oppositely charged ions
Why is magnesium used as the reducing agent in the extraction of titanium?
-Mg changes the oxidation state from 0 to 2+ so electrons are lost
-Ti changes oxidation state from 4+ to 0, so gains electrons
State the observations you would see when sodium hydroxide is mixed with
a) Magnesium chloride
b) Barium chloride
a) slight white precipitate
b) no visible change
Explain why the second ionisation energy of calcium is lower than the second ionisation energy of potassium
Ca+ looses electrons from a 4s orbital and k+ looses electrons from a 3p orbital, Ca looses from a higher energy level.
There is more shielding in Ca
What is the appearance of fluorine, chlorine, bromine and iodine?
Fluorine - pale yellow gas
Chlorine - pale green gas
Bromine - orange/brown liquid
Iodine - grey solid
Explain the trend in boiling point down group 7
Boiling point increases down the group due to:
-Increased size of atoms, increases the van de Waal forces
-More energy is needed to overcome these van de Waals
Physical state goes from gas at the top of the group, to solid at the bottom
Explain the trend in electronegativity down group 7
Electronegativity decreases down group 7 due to:
-Atoms getting larger, distance between positive nuclei and bonding electrons increases
-More shielding
Explain the trend in reactivity down group 7
Decreases down the group due to:
-Atoms with a larger radius attract electrons worse than smaller atoms, harder to gain an electron and react
Explain what occurs during a displacement reactions in group 7
Halogens are less oxidising down the group, we can show this by reacting halogens with halide ions
If Halide is lower in the periodic table than the halogen = displacement, halogen displaces halide from solution
a) Equation for displacement reaction of potassium bromide and chlorine
b) Ionic equation for displacement reaction of potassium bromide and chlorine
a) Cl2 + 2KBr ---> 2KCl + Br2
b) Cl2 + 2Br- ---> 2Cl- + Br2
K removed from equation as it is a spectator, not involved
As you can see, halogen (chlorine) displaces the halide (bromide ion), as Br is lower in the periodic table than Cl
Ionic equation for displacement reaction of potassium iodide and bromine
Br2 + 2I- --> 2Br- + I2
What is meant by a disproportionation reaction?
Reaction where an element is reduced and oxidised simultaneously
Equation for bleach
Sodium hydroxide + Chlorine --> Bleach + salt + water
2NaOH (aq) + Cl2(g) --> NaClO(aq) + NaCl(aq) +H2O(l)
NaCl = salt
NaClO = bleach (sodium chlorate I)
Why is the reaction of bleach a disproportionation reaction?
2NaOH (aq) + Cl2 (g) --> NaClO (aq) + NaCl (aq) + H2O (l)
0 +1 -1
Oxidation and reduction has occurred for chlorine
(reaction of chlorine with cold, dilute, aqueous NaOH)
Uses of sodium chlorate I, NaClO (bleach)
- Treating water
- Bleaching paper and fabrics
- Cleaning agents (bleach)
Reaction of chlorine and water
Forms hydrochloric acid and chlorate I acid
Cl2 (g) + H2O (l) -> HClO (aq) + HCl (aq)
0 +1 -1
Oxidation sate increases & decreases simultaneously =
disproportionation.
Chlorine kills bacteria in water
Explain why the chlorine in pools has to be replaced regularly?
Sunlight decomposes chlorinated water, can no longer kill bacteria
2H20(l) + 2Cl(g) --> 4HCl(aq) + O2 (g)
Evaluate the use of drinking chlorinated water
Advantages:
-Destroys microorganisms that cause diseases
-Long lasting, reduced bacteria build up further down the supply
-Reduces the growth of algae that discolours the water & gives it a bad taste and smell
Disadvantages:
-Chlorine gas is toxic and irritates the respiratory system
-Liquid chlorine causes severe chemical burns
-Chlorine can react with organic compounds to make chloroalkanes , linked with causing cancer
However if you do not chlorinate water, risk of cholera epidemic
Why are halides good reducing agents?
Loose (donate) electrons, itself gets oxidised
Why is a I- ion more powerful than a F- ion?
Reducing power of halides increases down a group.
-Ionic radius increases, more shielding and distance between nucleus and outer shell electrons, force is weaker
-Outer electron is lost more readily
Displacement reactions which make a dark brown solution
potassium iodide + chlorine
potassium iodide + bromine
React a few drops of CONCENTRATED sulfuric acid (H2SO4) with sodium chloride. Chloride ions are not strong enough reducing agents to reduce sulfuric acid. Instead you just produce hydrogen chloride and sodium hydrogen sulfate
a) State your observations
b) Write a symbol equation to show this reaction
c) Deduce the oxidation state of sulfur in sulfuric acid and sodium hydrogen sulfate
a) -No green chlorine
-White fume
- Effervescence
b) H2S04 + NaCl ---> HCl + NaHSO4
c) +6 in sulfuric acid, +6 in sodium hydrogen sulfate
React a few drops of CONCENTRATED sulfuric acid with sodium bromide. (redox) Bromide ions are strong enough reducing agents to reduce sulfuric acid to make sulfur dioxide. Bromine ions themselves are oxidised to form bromine gas.
a) State your observations
b) Write two half equations to show these reactions
c) Construct an overall equation for this reaction
d) Deduce the oxidation state in sulfur dioxide
a) Dark orange vapour
White fumes
b) 2Br- ---> Br2 + 2e- (oxidation of bromide ion)
H2SO4 + NaBr --> SO2
Balance the equation
H2S04 + NaBr + 2H+ + 2e- --> SO2 + 2H20 (being reduced)
c) electrons must be equal to combine (both 2e- so that's fine), cancel out e-.
H2S04 + 2Br + NaBr + 2H+ --> Br2 + SO2 + 2H20
d) +4
Sulphur dioxide produced is a choking gas
React a few drops of CONCENTRATED sulfuric acid with sodium iodide. Iodide ions are strong enough reducing agents to reduce sulfuric acid to SO2, S and H2S.
1.State your observations
2. Write half equations for
a) SO2
b) S
C) H2S
1. Rotten egg smell from H2S, white flames, violet vapour, yellow solid
2. a) H2S04 + 2H+ + 2e- --> SO2 + 2H20
b) H2S04 +6H+ +6e- --> S +4H20
c) H2S04 +8H+ 8E- -> H2S + 4H20
Iodide ions are themselves oxidised to form iodine gas. Write a half equation to show this and combine it with the 3 equations listed
a) H2S04 + 2e- + 2H+ --> SO2 + 2H20
b) H2S04 + 6H+ 6E- --> S + 4H20
c) H2S04 + 8H+ + 8E --> H2S + 4H20
2I- --> I2 + 2e- (oxidation of iodide ions)
a) electrons in both equations are the same, 2e- no need to balance simply cancel out electrons
H2S04 + 2H+ + 2I- --> I2 + SO2 + 2H20 (sulfur being reduced)
+6 +4
b) electrons in equations are not equal, x3 oxidation of iodide ions to = 6e-
6I- --> 3I2 + 6e- (iodide ions being oxidised)
H2S04 + 6H+ + 6I- --> 3I2 + S + 4H20
+6 0
c) electrons in equations are not equal x4 oxidation of iodide ions to = 8e-
8I- --> 4I2 + 8e- (iodide ions being reduced)
H2S04 + 8H+ + 8I- --> 4I2 + H2S + 4H20 (sulfur being reduced)
+6 -2
Test for halides using silver nitrate, confirm this test using ammonia
1. Add dilute nitric acid first, so the nitric acid reacts with any anions other than halides (carbonates), stops false positive test occurring
2. Add silver nitrate solution (AgNO3), the colour of the precipitate helps identify the halide ion
F- = No precipitate, silver fluoride is soluble in water
Cl- = White precipitate
Br- = Cream precipitate
I- = yellow precipitate
2. Confirm using ammonia (NH3)
Cl- = White precipitate dissolves in dilute NH3 (colourless solution)
Br- = Cream precipitate dissolves in concentrated NH3 (colourless solution)
I- = Yellow precipitate insoluble in NH3 (yellow precipitate remains)
Ionic equation format for reaction of halide ion with silver nitrate
Ag+ + X- --> AgX
Where X is Cl, Br or I
Example: Ag+ + Cl- --> AgCl
An element in Period 3 forms an oxide that is insoluble in water.This oxide reacts with sulfuric acid and with aqueous potassium hydroxide.
Give the formula for this oxide.Give an equation for the reaction of this oxide with sulfuric acid (2)
Formula Al2O3
Equation Al2O3 + 3H2SO4 ---> Al2(SO4)3 + 3H20
Give an equation, including state symbols, to represent the process that occurs when the third ionisation energy of sodium is measured
Na2+(g) → Na3+(g) + e−
Phosphorus burns in air to form phosphorus(V) oxide. Give an equation for this reaction
4P + 5O2 → P4O10
Amphoteric meaning
If its able to react as a base and as an acid
Explain why sodium oxide forms an alkaline solution when it reacts with water
Sodium oxide contains O2- ionsThese O2- ions react with water forming OH- ions
Write an equation for the reaction of sodium oxide with water
Na2O + H2O → 2NaOH
The Ne atom and the Mg2+ ion have the same number of electrons. Give two reasons why the first ionisation energy of neon is lower than the third ionisation energy of magnesium.
-Mg2+ ion smaller than Ne atom
-Mg2+ has a higher nuclear charge
The elements phosphorus, sulfur, chlorine and argon are in the p block of the Periodic Table.
State why these elements are classified as p block elements.
Outer electrons in a p orbital
In terms of atomic structure, explain why the van der Waals' forces in liquid argon are very weak
Lacks permanent dipoles
Give an equation for the reaction of solid sodium bromide with concentrated sulfuric acid to form bromine
2H2SO4 + 2NaBr → Na2SO4 + SO2 + Br2 + 2H2O
A solution that is thought to contain chloride ions and iodide ions is tested.
Dilute nitric acid is added to the solution.
Aqueous silver nitrate is added to the solution.
A pale yellow precipitate forms.
Excess dilute aqueous ammonia is added to the mixture.
Some of the precipitate dissolves and a darker yellow precipitate
remains.
Give a reason for the use of each reagent.
Explain the observations.
Give ionic equations for any reactions
Dilute nitric acid: removes other ions that may give ppts with AgNO3
AgNO3 produces ppts with halides
NH3 dissolves AgCl
Write an equation to show how solid potassium fluoride reacts with concentrated sulphuric acid
KF + H2SO4 → KHSO4 + HF
Write an equation for the redox reaction of sodium bromide with concentrated sulphuric acid
2 H2SO4 + 2 Br- → SO2 + Br2 + 2 H2O + SO42-
Identify the two halides which do not reduce concentrated sulphuric acid. Write an equation for the reaction which does occur with one of these two halides
Flouride and chloride
H+ + F- → HF
Magnesium burns with a bright white light and is used in flares and fireworks. Use your knowledge of the reactions of Group 2 metals with water to explain why water should not be used to put out a fire in which magnesium metal is burning
H2 produced, H2 is explosive
Write the simplest ionic equation for the reaction that occurs between magnesium chloride and sodium hydroxide
Mg2+(aq) + 2OH-(aq) → Mg(OH)2(s)
Calcium ethanoate, (CH3COO)2Ca, is used in the treatment of kidney disease. Thermal decomposition of calcium ethanoate under certain conditions gives propanone and one other product
Write an equation for the thermal decomposition of calcium ethanoate
(CH3COO)2Ca → CH3COCH3 + CaCO3
Concentrated sulfuric acid reacts with solid sodium chloride and with solid sodium bromide
State one similarity and one difference between these reactions
Similarity: forms hydrogen halides/misty white fumes/forms sodium sulfate/forms sodium hydrogensulfate/effervesence
Difference: Br2 produced with solid sodium bromide/bromide undergoes redox
Separate unlabelled solid samples of three anhydrous sodium compounds are provided for a student to identify. These compounds are known to be sodium carbonate, sodium fluoride and sodium chloride but it is not known which sample is which. Outline a logical sequence of test-tube reactions that the student could carry out to identify each of these compounds. Include the observations the student would expect to make. Give equations, including state symbols, for any reactions that would take place.
Add nitric acid to all three, add water to make a solution
Add AgNO3
Na2CO3 will fizz with acid
NaCl gives a white precipitate
NaF will show no visible change
Na2CO3 + 2HNO3 ---> 2NaNO3 + CO2 + H2O
AgNO3 + NaCl---> AgCl + NaNO3
Cl2 + H2O ⇌ HClO + HCl
Give two half-equations to show the oxidation and reduction processes that occur in this redox reaction.
Oxidation: Cl2 + 2H2O --> 2HClO + 2H + 2e-
Reduction: Cl2 + 2H+ 2e- ---> 2HCl
When aqueous sodium thiosulphate is added to solid silver bromide a reaction occurs and a colourless solution is formed.
Identify the silver-containing species present in the colourless solution
[Ag(S2O3)2]3-;
How can the addition of an aqueous solution of chlorine be used to distinguish between aqueous solutions of sodium bromide and sodium iodide?
State any observations you would make and write equations for the reactions occurring
Cl2 to Br- results in yellow-brown solution
2Br- +Cl2 →2Cl- +Br2
Cl2 to I2- results in brown/black solution or grey solid
2I- +Cl2 →2Cl- +I2
How can reactions with concentrated sulphuric acid be used to distinguish between solid samples of sodium bromide and sodium iodide?
State the observations you would make and give all the oxidation and reduction products formed in both reactions. Using half-equations, construct an overall equation for one of these redox reactions. (11 marks_
Bromine: dark orange vapour
Br2 and SO2 produced
2Br- ---> Br2 + 2e-
Iodide: violet fumes/grey solid
Iodine, S, H2S and SO2 produced
2I- ---> I2 + 2e-
State what you would observe when chlorine gas is bubbled into an aqueous solution of potassium iodide. Write an equation for the reaction that occurs
Brown solution
Cl2 + KI --> 2KCl + I2
a) How does reducing ability change down group 7 and why
b) How does oxidising ability change down group 7 and why
a) Increases, as atomic radius and shielding increases, the outer electron is further from the nucleus and hence electrons are easier to remove
b) Decreases oxidation is the accepting of electrons, so higher atomic radius and more shielding makes it harder to gain an electron