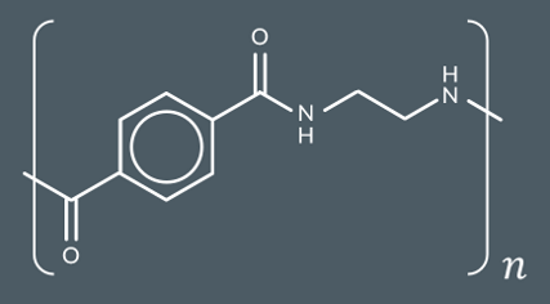
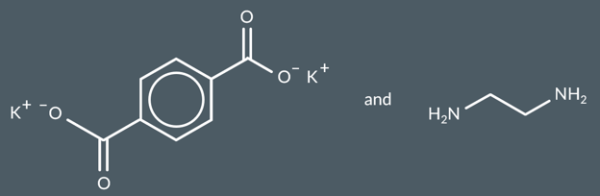
condensation polymers
1/56
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
57 Terms
Under certain conditions, addition polymers are formed from...
alkenes.
During addition polymerisation, each alkene...
loses a double bond
forms a single bond to two other carbons
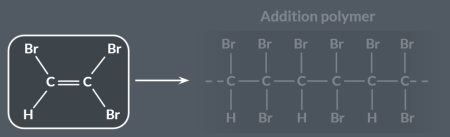
We call this alkene a…
monomer.
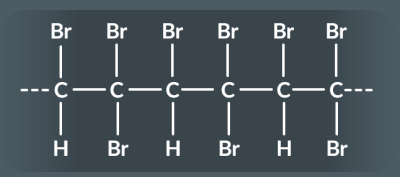
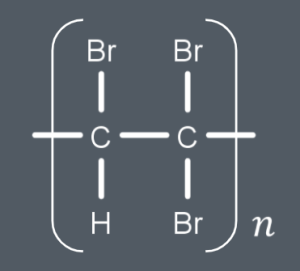
What’s the repeat unit of this polymer?
When we react a carboxylic acid with an alcohol, we produce…
an ester and water
Draw the organic product of this reaction.

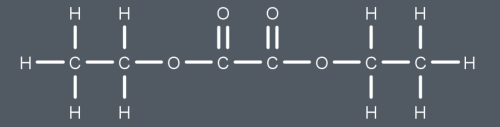
What do we produce from this reaction?
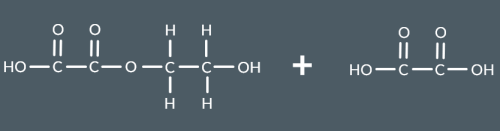
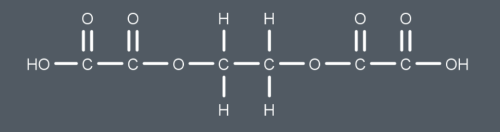
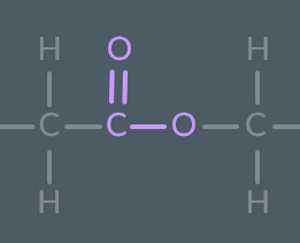
What is this functional group called?
ester
Dicarboxylic acids and diols react to form…
a polyester and water
Polyesters are polymers that contain…
ester links
What’s the repeat unit for this polyester?
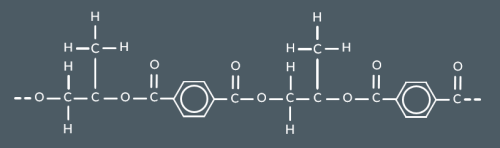
The repeat unit for a polyester made from a dicarboxylic acid and diol contains...(4)
Two carbonyl groups
Two oxygen groups
The atoms between two oxygens
The atoms between two carbonyl groups
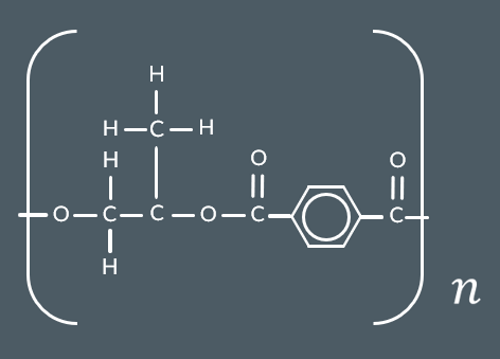
What’s the repeat unit for this polyester?
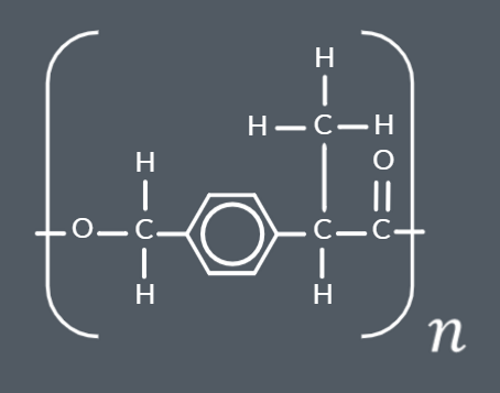
Polyesters can be made from a single monomer containing…
a carboxylic group and an alcohol group
The repeat unit for a single monomer polyester contains...(3)
a single carbonyl group
a single oxygen atom
the atoms between oxygen and the carbonyl group.
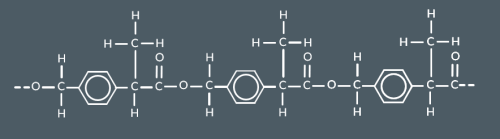
Which polyester forms from these monomers?
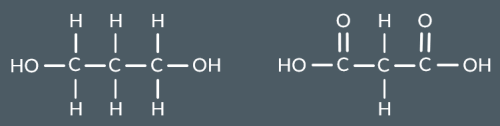
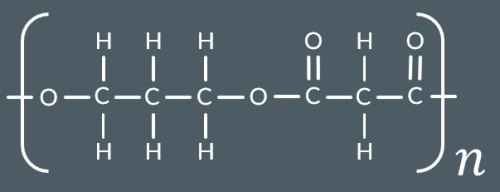
Which polyester forms from these monomers?
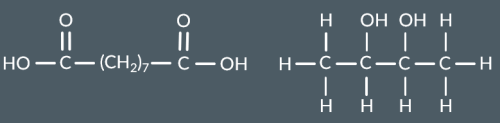
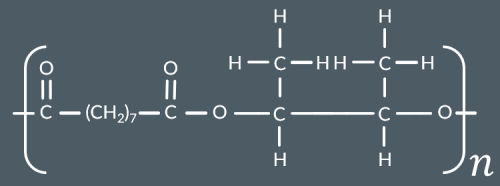
Which polyester forms from this monomer?
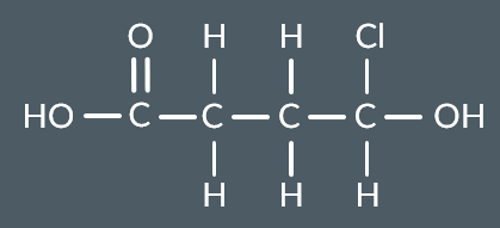
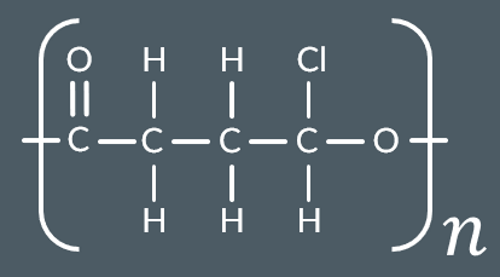
Which polyester forms from these monomers?
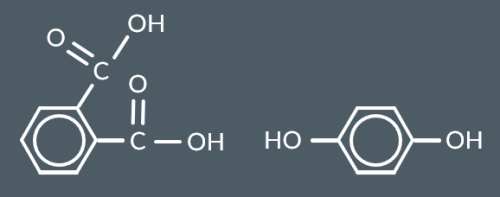
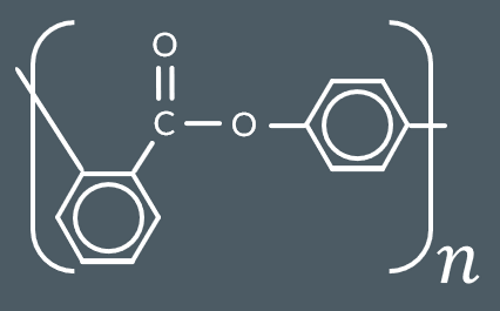
Which polyester forms from this monomer?
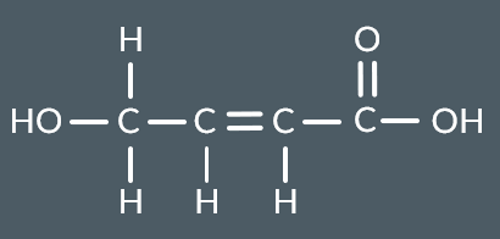
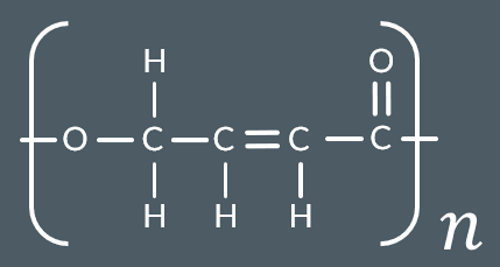
What do we produce in this reaction?

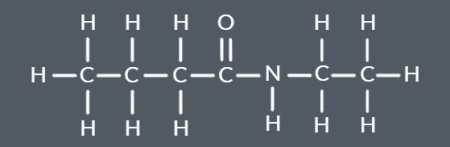
A carboxylic acid and an amine react to produce…
amide and water
We call a molecule with two amine groups a…
diamine
In the reaction to form a polymer, the dicarboxylic acid and the diamine are the…
monomers
Which polymer forms from these monomers?

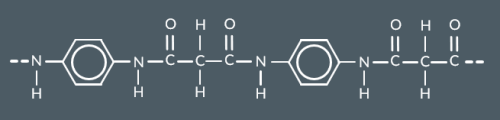
Diamines and dicarboxylic acids react to form…
a polyamide and water
Polyamides are polymers that contain…
amide links
What’s the repeat unit for this polyamide?
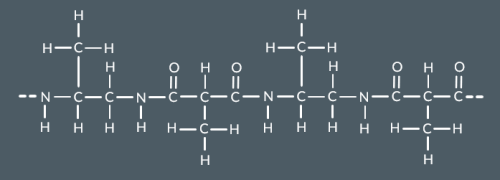
The repeat unit for a two monomer polyamide contains...(4)
Two NH groups
Two carbonyl groups
The atoms between two NH groups
The atoms between two carbonyl groups
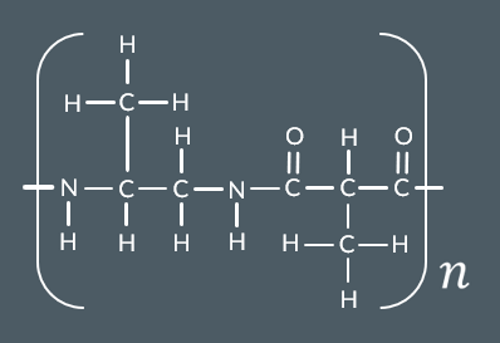
What polyamide forms from this monomer?
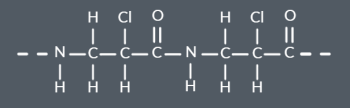
What is the repeat unit for this single monomer polyamide?
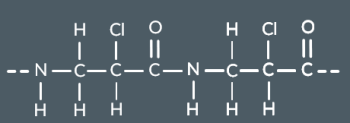
Polyamides can be made from a single monomer containing…
a carboxylic acid and amine functional group
The repeat unit for a single monomer polyamide contains...
A single carbonyl group
A single NH group
The atoms between NH group and the carbonyl group
Which polyamide forms from these monomers?
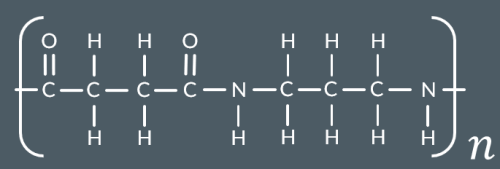
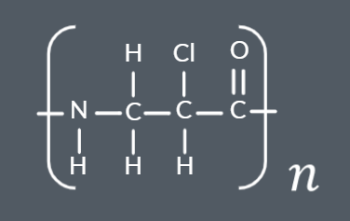
Which polyamide forms from this monomer?
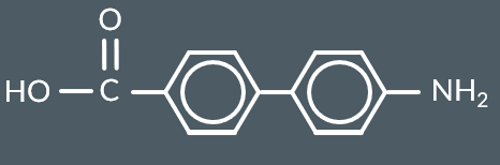
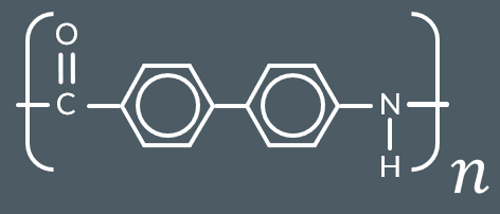
Which polyamide forms from these monomers?
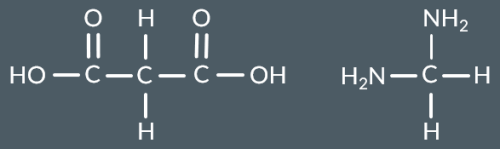
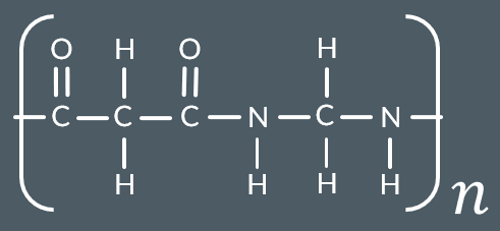
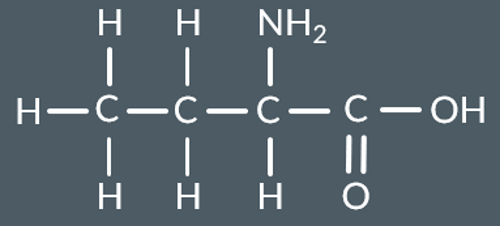
Which polyamide forms from this monomer?
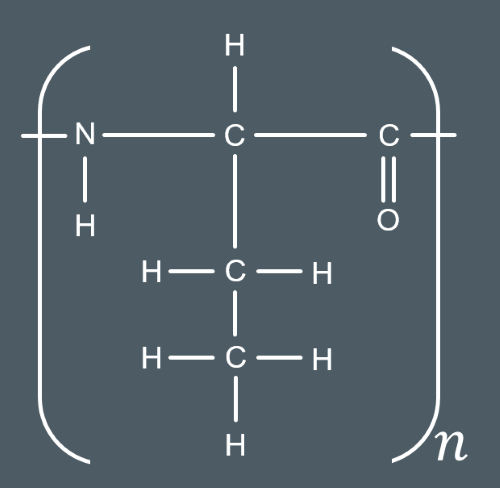
What type of polymers are Polyesters and polyamides examples of?
condenstation polymers.
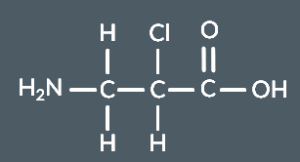
Identify the products that form from this monomer.
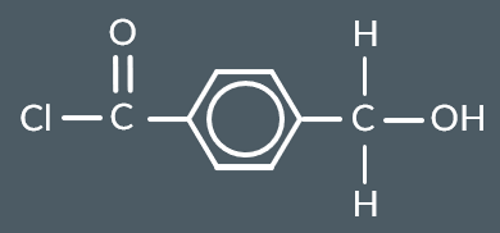
and HCl
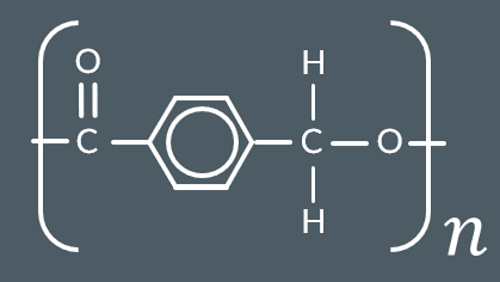
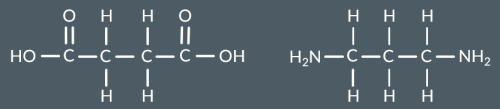
Identify the condensation polymer that forms from these monomers.
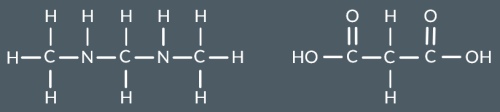
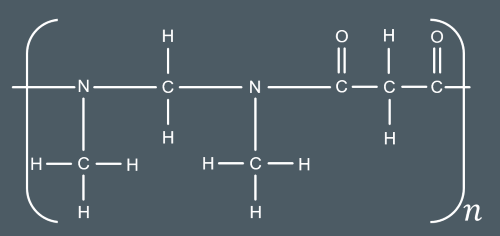
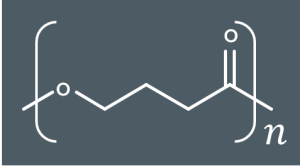
2)Molecule A can also lose HCl to form a cyclic molecule, B. Suggest a structure for molecule B.
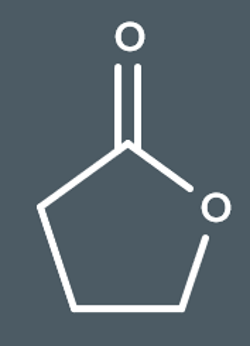
This polyester is formed from…
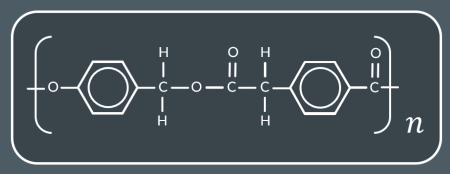
how many monomers?
2
If one of the monomers is a diacyl chloride, instead of adding OH to the carbonyl group, we need to add…
Cl
Identify the monomer(s) that formed this polymer.
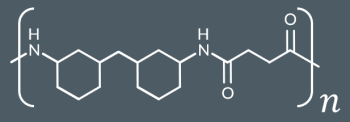
Break the amide link.
2. 2. Separate out the atoms.
3. 3. Add H to both NHs and add OH to both C=Os.
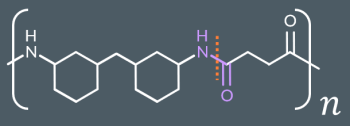
Identify the monomer(s) that formed this polymer.
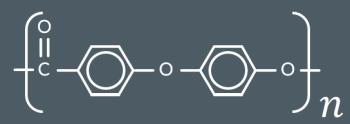
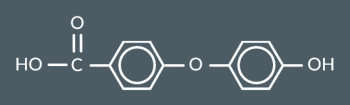
Draw a monomer or pair of monomers that could have formed this polymer.
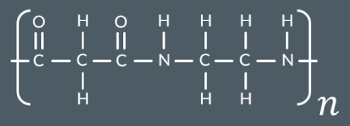
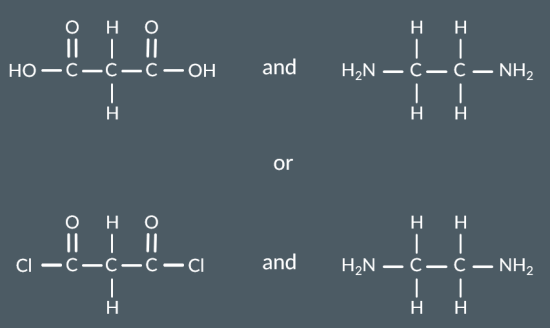
Draw a monomer or pair of monomers that could have formed this polymer.
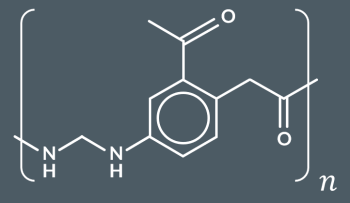
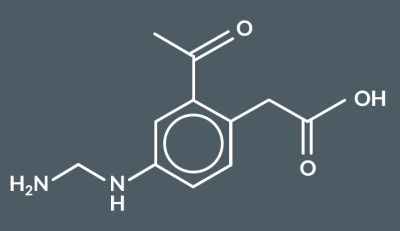
What type of reaction occurs when a polyester reacts with water?
hydrolysis reaction
When a polyester reacts with water which group breaks and forms…(3)
ester links break.
COOH groups form
OH groups form
This hydrolysis reaction requires…
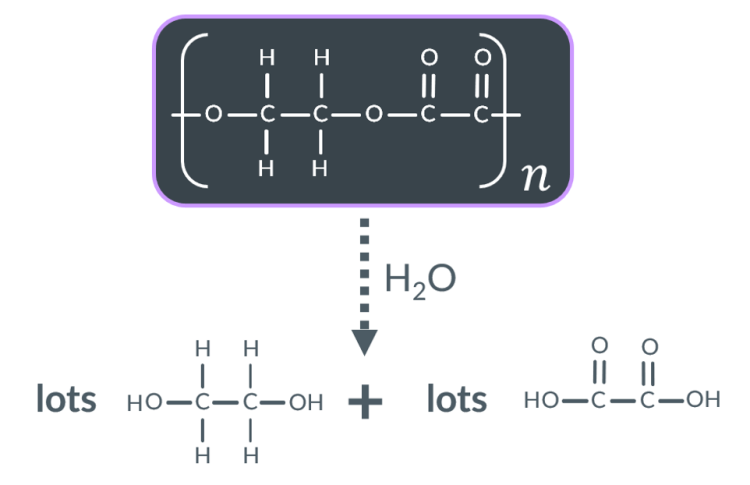
an acid catalyst.
What forms when a polyamide undergoes acid hydrolysis, …
NH3+ groups form.
COOH groups form
What do we form when this polyamide undergoes base hydrolysis?
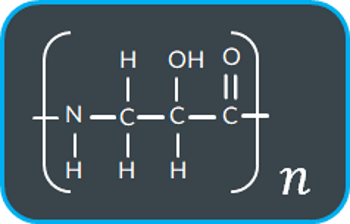
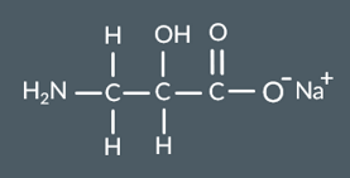
When a polyester undergoes base hydrolysis with NaOH (aq) what groups form?…
OH groups form.
COO- Na+ groups
When a polyamide undergoes base hydrolysis with NaOH (aq) what groups form?…
NH2 groups form
COOH groups form
Select the products formed when this polyester is reacted with excess dilute NaOH (aq).
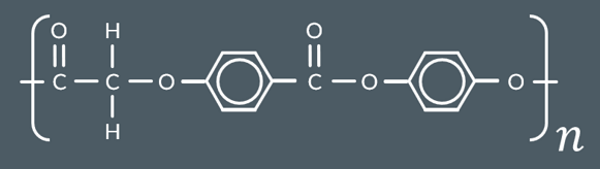
When a polyester undergoes base hydrolysis, the ester links break, the alcohol groups reform, and the carboxylate groups form salts with Na+ ions.
And Hexagon with OH groups
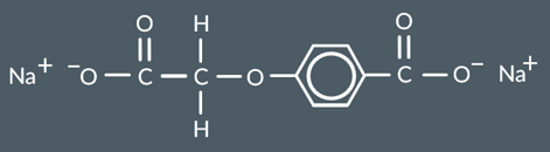
Complete the structure of the product formed when this polymer undergoes acid hydrolysis.
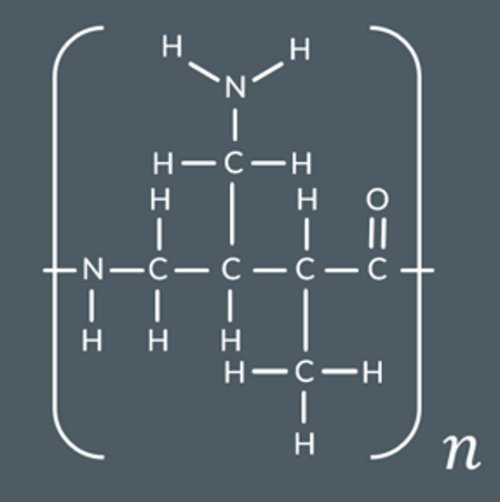
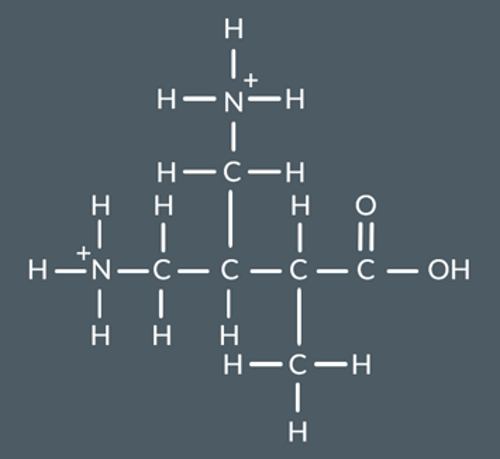
Draw the products formed when this polymer is hydrolysed with excess aqueous KOH.