Molecules of life (bonds)
1/7
Earn XP
Description and Tags
working through checklist
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
Orbital Hybridisation - what is it
mixing of atomic orbitals to form new hybrid orbitals.
hybrid orbitals have different shapes and energies than the original atomic orbitals.
used to explain geometry of molecules and the types of bonds that can be formed
Orbital Hybridisation - process
atoms bond together to form a molecule
atomic orbitals overlap to create new hybrid orbitals that can accommodate the shared electrons
The type of hybridization that occurs depends on the number and types of orbitals involved in the bonding.
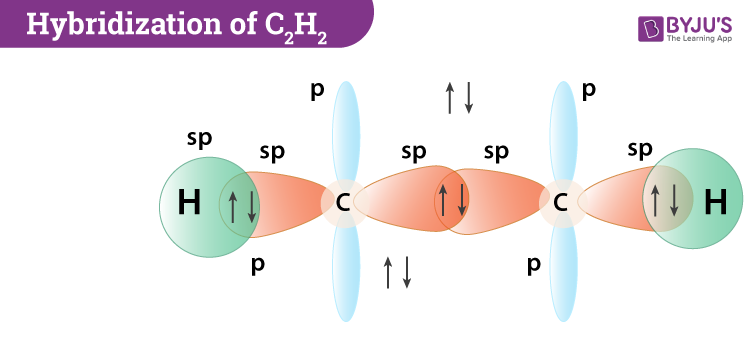
states of different hybridizations - sp
one s orbital and one p orbital are combined to form two hybrid orbitals
180 degrees.
CH=CH
linear
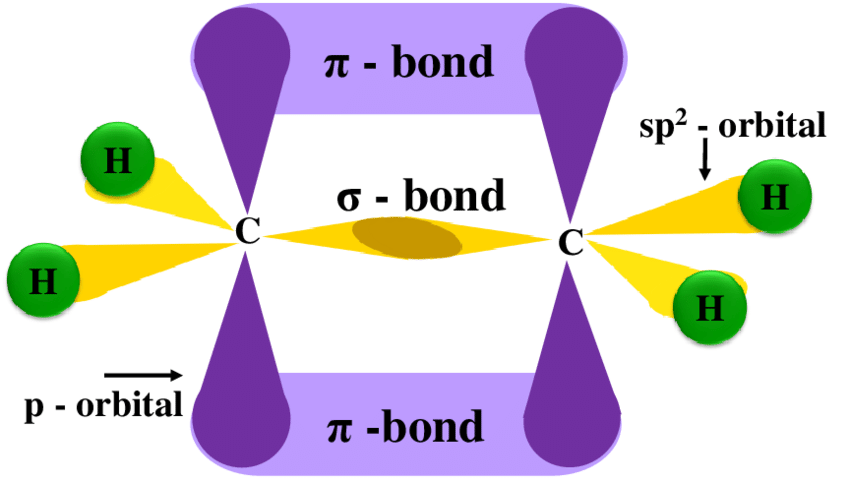
states of different hybridizations - sp2
one s orbital and 2 p orbitals are combined to form three hybrid orbitals
120 degrees
Ch2=CH2
triagonally
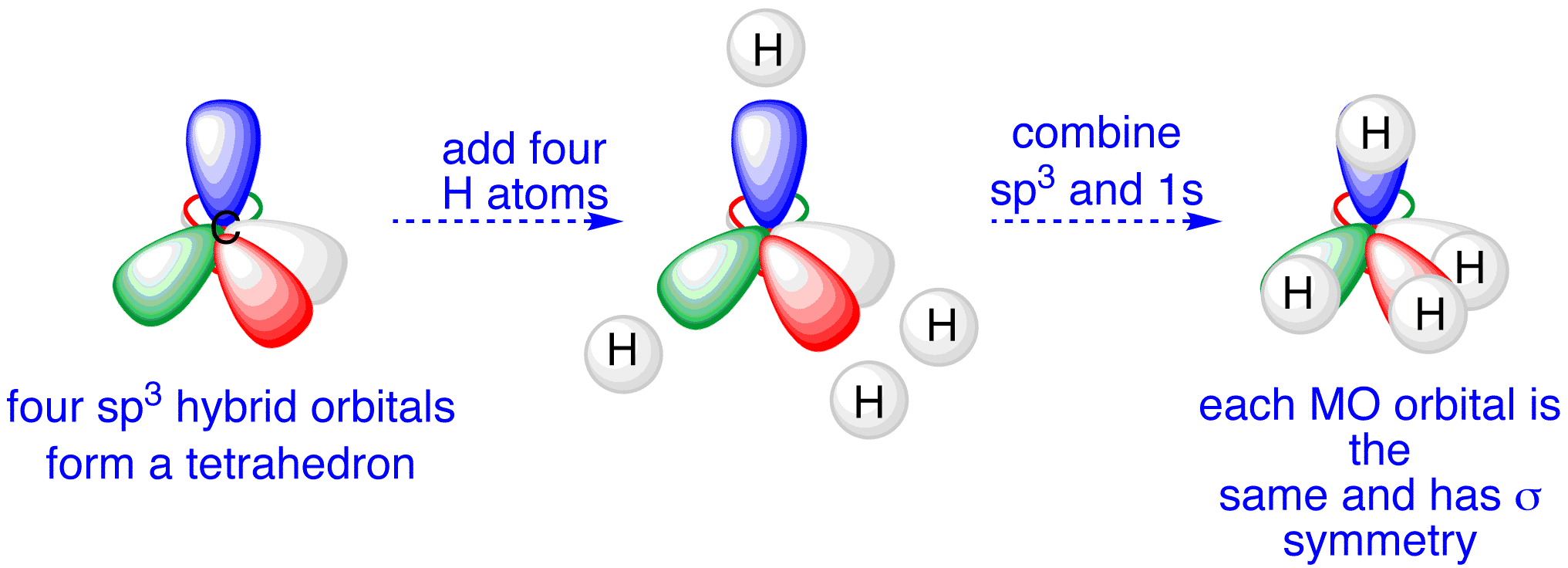
states of different hybridizations - sp3
one s orbital and three p orbitals are combined to form four hybrid orbitals
109.5 degrees
tetrahedral
CH4
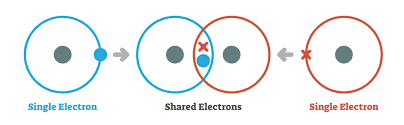
Covalent bonds and properties
Forms when two atoms share one or more pairs of electrons to achieve stable elec config (non metal atoms)
Both atoms contribute one or more electrons to form a shared electron pair in the region of space between two nuclei
Electrons are attracted to both nuclei, holding the two atoms together in a stable molecule
sharing lowers the energy state of both atoms compared to their isolated states.
Polarity of Covelent Bonds (non polar and polar)
NON- polar = electrons shared equally - no dipole movement - no significant charge separation (example H2, O2)
POLAR - electrons shared unequally - dipole movement and partial charge separation
One atom attracted shared electronsg stronger than other
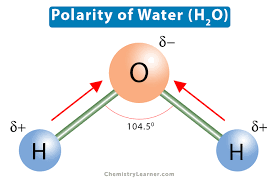
Water (H20) - oxygen attracts shared electrons more strongly than hydrogen so partial negative charge on oxygen and partial positive charge on hydrogen.
Covalent - Sigma bonds