MCAT Physics and Math - Reasoning About the Design and Execution of Research
1/65
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
66 Terms
scientific method
set of steps that defines the appropriate order of events to structure and carry out an experiment; established protocol for transitioning from a question to a new body of knowledge
steps in the scientific method
Generate a testable question
Gather data and resources
Form a hypothesis
Collect new data
Analyze the data
Interpret the data and existing hypothesis
Publish
Verify results
hypothesis
proposed explanation or proposed answer to our testable question; often in the form of an if–then statement
experimentation
involves manipulating and controlling variables of interest
observation
often involves no changes in the subject’s environment
peer review
evaluation of work by one or more people with similar competencies as the producers of the work
FINER method
method to determine whether the answer to one’s question will add to the body of scientific knowledge in a practical way and within a reasonable time period
FINER Method question
feasible?
interesting?
novel?
ethical?
relevant?
Basic science research
kind conducted in a laboratory, and not on people; generally the easiest to design because the experimenter has the most control; causal relationship is being examined because the hypothesis generally states a condition and an outcome
control / standard
a method of verifying results; an experiment or observation designed to minimize the effects of variables other than the independent variable
Positive controls
ensure a change in the dependent variable when it is expected
Negative controls
ensure no change in the dependent variable when no change is expected
placebo effect
observed or reported change when an individual is given a sugar pill or sham intervention
independent variable
variable that is manipulated
dependent variable
variable(s) that are measured or observed
causal
If the change in the independent variable always precedes the change in the dependent variable, and the change in the dependent variable does not occur in the absence of the experimental intervention
Accuracy / validity
the ability of an instrument to measure a true value
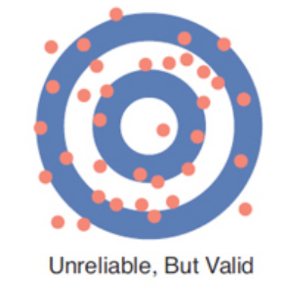
Precision / reliability
ability of the instrument to read consistently, or within a narrow range

systematic error
affects measurements by the same amount or proportion, provided that a reading is taken the same way each time; affects accuracy but not precision; only an inaccurate tool will introduce bias
random error
causes one measurement to differ slightly from the next; changes precision, not accuracy; accuracy; usually overcome by using a large sample size
human subjects research
systematic, scientific investigation that can be either interventional or observational and involves human beings as research subjects
Randomization
method used to control for differences between subject groups in biomedical research; uses an algorithm to determine the placement of each subject into various experimental groups or controls
blinded (experiment)
do not have information about which group the subject is in
single-blind experiments,
only the patient or assessor is blinded
assessor
the person who makes measurements on the patient or performs subjective evaluations
double-blind experiments
investigator, subject, and assessor all do not know the subject’s group
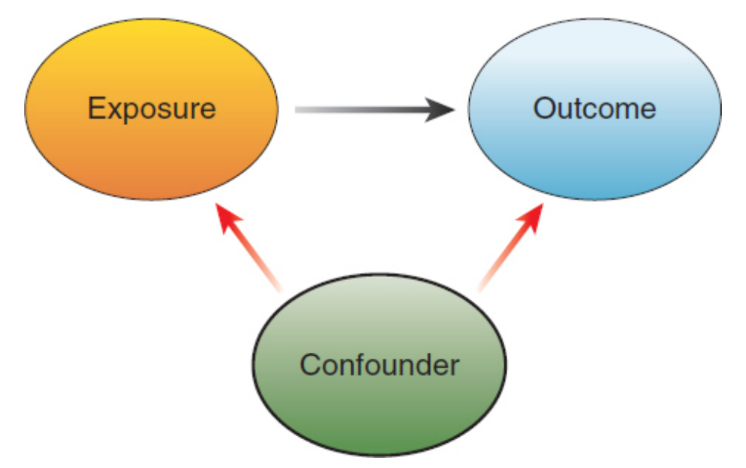
confounding variable / confounders
variables outside of the independent and dependent variables considered that could affect results
binary variables
variables of two choices
ex. yes v. no, better v. worse
continuous variables
variables that any given number can fulfill, sometimes including fractions, decimals and negatives
ex. amount of weight lost, percent improvement in cardiac output
categorical variables
variable that can take on one of a limited, and usually fixed, number of possible values
ex. state of residence, socioeconomic status
Regression analysis
a set of statistical processes for estimating the relationships between a dependent variable and one or more error-free independent variables; may demonstrate linear, parabolic, exponential, logarithmic, or other relationships
Observational studies
draw on the available data and analyze it
Cohort studies
subjects are sorted into groups based on differences in risk factors and then assessed at various intervals to determine how many subjects in each group had a certain outcome
exposures
risk factors in a correlational study; independent variable
outcome
measured results of a correlational study; dependent variable
Cross-sectional studies
categorize patients into different groups at a single point in time
Case-control studies
identifying the number of subjects with or without a particular outcome, and then look backwards to assess how many subjects in each group had exposure to a particular risk factor
Hill’s criteria (definition)
describe the components of an observed relationship that increase the likelihood of causality in the relationship
Hill’s criteria (list)
Temporality: The exposure must occur before the outcome
Strength: As more variability in the outcome variable is explained by variability in the study variable, the relationship is more likely to be causal.
Dose–response relationship: As the study or independent variable increases, there is a proportional increase in the response.
Consistency: The relationship is found to be similar in multiple settings.
Plausibility: There is a reasonable mechanism for the independent variable to impact the dependent variable supported by existing literature.
Consideration of alternative explanations: If all other plausible explanations have been eliminated, the remaining explanation is more likely.
Experiment: If an experiment can be performed, a causal relationship can be determined conclusively.
Specificity: The change in the outcome variable is only produced by an associated change in the independent variable.
Coherence: The new data and hypothesis are consistent with the current state of scientific knowledge.
correlation
any statistical relationship, whether causal or not, between two random variables or bivariate data
Bias
result of flaws in the data collection phase of an experimental or observational study
Confounding
error during analysis; data may or may not be flawed, but an incorrect relationship is characterized
selection bias
subjects used for the study are not representative of the target population
Detection bias
educated professionals using their knowledge in an inconsistent way
Hawthorne effect / observation bias,
the behavior of study participants is altered simply because they recognize that they are being studied
beneficence
obligation to act in the patient’s best interest
nonmaleficence
obligation to avoid treatments or interventions in which the potential for harm outweighs the potential for benefit
autonomy
responsibility to respect patients’ decisions and choices about their own healthcare
justice
responsibility to treat similar patients with similar care, and to distribute healthcare resources fairly; applies to both the selection of a research topic and the execution of the research; when there is risk associated with a study, it must be fairly distributed so as not to unduly harm any group
Belmont Report
landmark document published by the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research in 1979, delineates the three necessary pillars of research ethics:
respect for persons
justice
(slightly more inclusive version of) beneficence
Respect for persons
the need for honesty between the subject and the researcher, and generally—but not always—prohibits deception; includes the process of informed consent; Confidentiality
informed consent
a patient must be adequately counseled on the procedures, risks and benefits, and goals of a study to make a knowledgeable decision about whether or not to participate in the study without coercive influence
institutional review boards
group that has been formally designated to review and monitor ethical research involving human subjects
Vulnerable persons
require special protections above and beyond those taken with the general population
ex. children, pregnant individuals, and prisoners
Confidentiality
keeping information about subjects secret or private
Tuskegee syphilis experiment
notorious forty-year study (1932–1972) by the United States Public Health Service that was fraught with extreme violations of the ethical principle of respect for persons; considered the primary impetus for the writing of the Belmont Report
African American men living in conditions of poverty were enrolled into a study on the natural progression of syphilis. These men were given sham treatments, barred from accessing appropriate healthcare, and repeatedly deceived by investigators—including the fact that they were never told they had syphilis!
Morally relevant differences
differences between individuals that are considered an appropriate reason to treat them differently
equipoise
a situation in which things are perfectly balanced; one cannot approach research with the knowledge that one treatment is superior to the other or the trial must be stopped because providing an inferior treatment is a net harm
population
complete group of every individual that satisfies the attributes of interest
parameter
Information that is calculated using every person in a population
sample
any group taken from a population that does not include all individuals from the population
statistic
Information about a sample
internal validity
support for causality
external validity / generalizability
samples that are representative of the target population
statistically significant
not the result of random chance
clinical significance
notable or worthwhile change in health status as a result of intervention