NC State Chemistry Placement Topics
1/99
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
100 Terms
Stoichiometry
deals with the relationships between the elements making up substances and the property of the substances
Law of Definite Proportions
deals with chemical compounds, or substances that consist of two or more elements; states that the element of every pure compound exist in the same ratio by mass
Law of Multiple Proportions
states that when chemical elements combine in a chemical reaction to form a compound, they combine in a ratio of small whole numbers
Avagadro's number
one mole (mol) = 6.02 x 10^23
Periodic Table
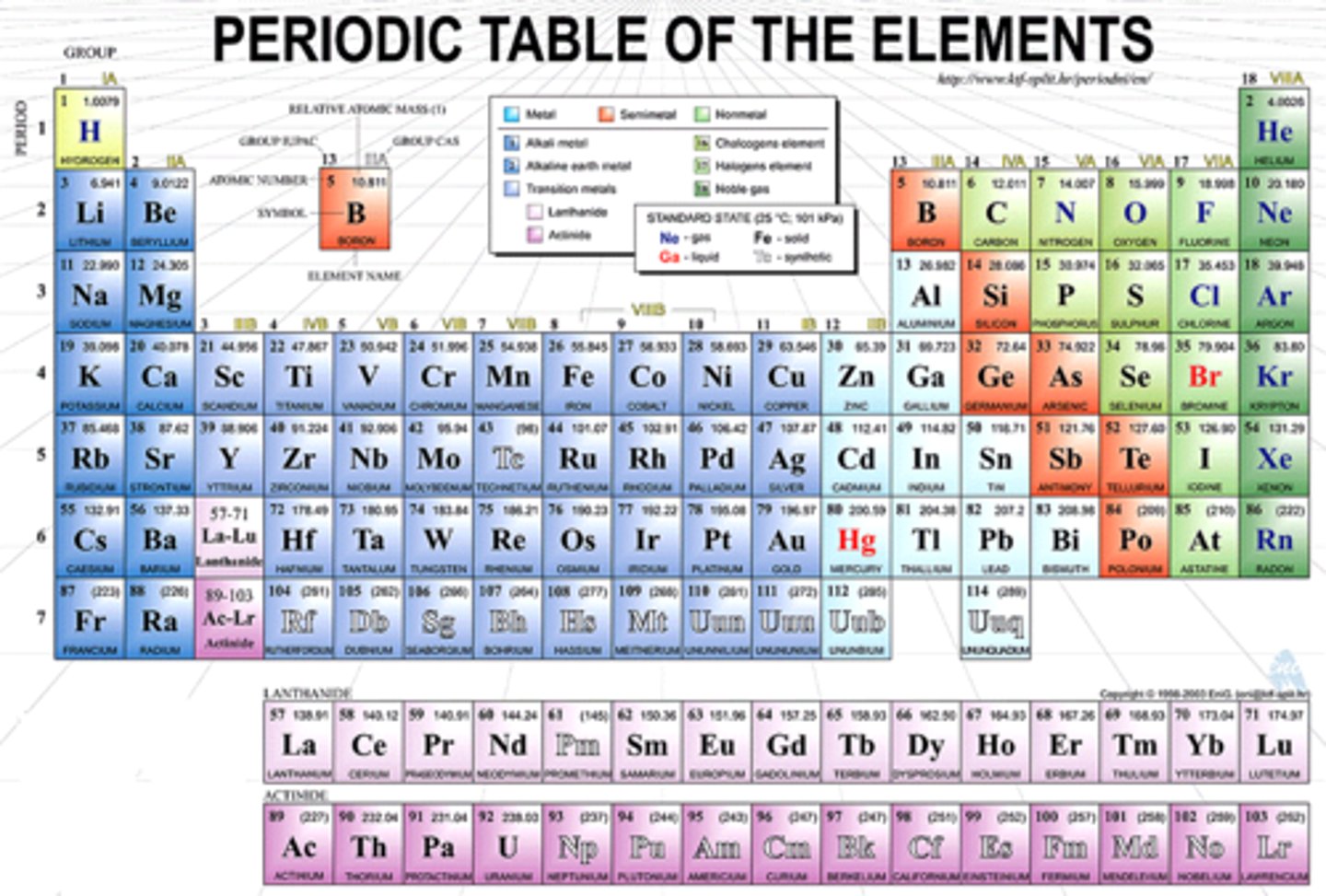
Periodic Table Trends
Moving Left to Right: atomic radius decreases, ionization energy increases, electronegativity increases.
Moving TOP to BOTTOM: atomic radius increases, ionization energy decreases, electronegativity increases
Gas Laws
experiments with a large number of gases reveal that the state, or condition, of many gaseous substances can be defined using four variables: temperature (T), pressure (P), volume (V), and the quality
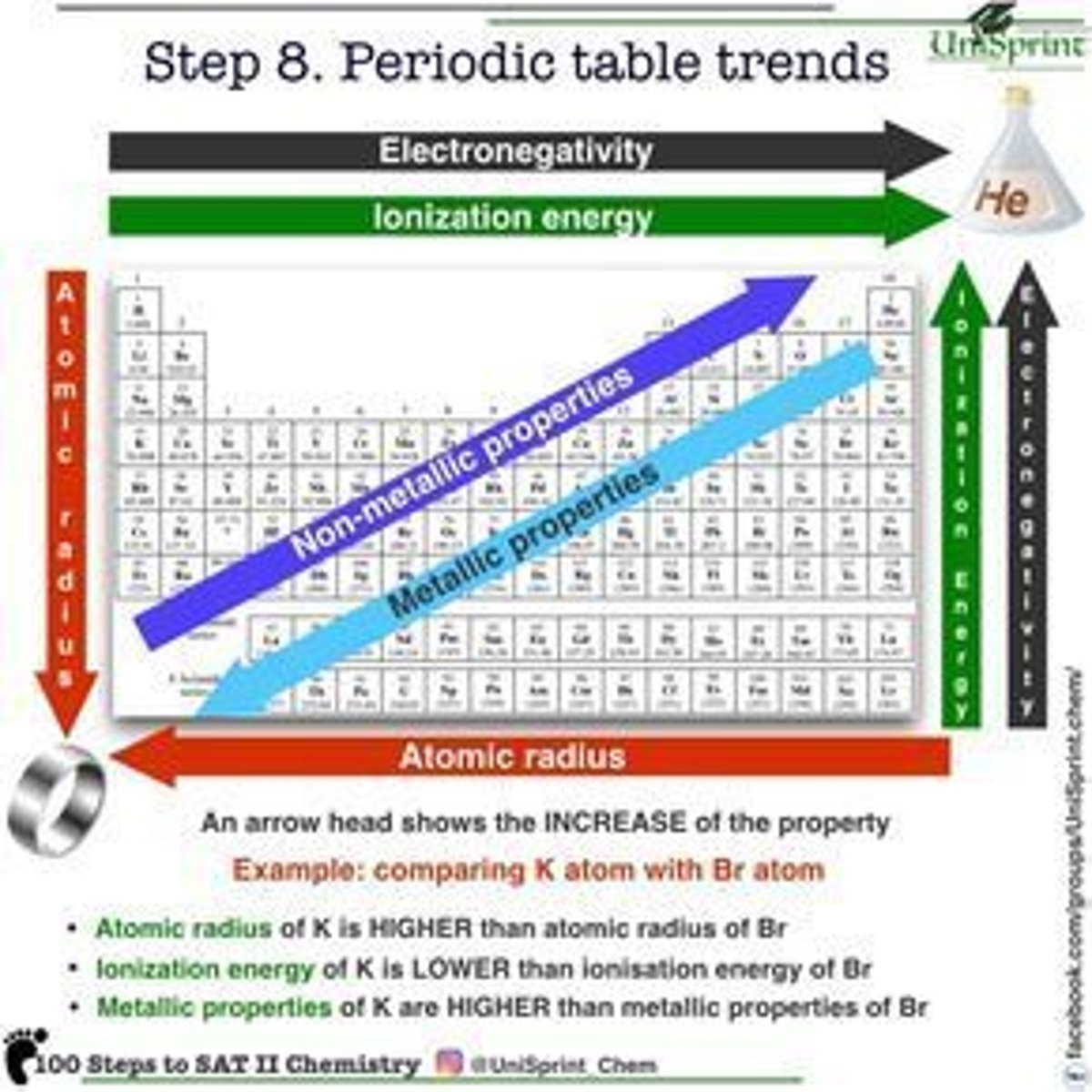
Mass
Kilogram (kg)
Length
Meter (m)
Time
Second (s or sec)
Temperature
Kelvin (K)
Amount of substance
Mole (mol)
Electric current
Ampere (A or amp)
luminous intensity
Candela (cd)
Peta (P)
10^15
Tera (T)
10^12
Giga (G)
10^9
Mega (M)
10^6
Kilo (k)
10^3
Deci (d)
10^-1
Centi (c)
10^-2
Milli (m)
10^-3
Micro (μ^b)
10^-6
Nano (n)
10^-9
Pico (p)
10^-12
Femto (f)
10^-15
Atto (a)
10^-18
Zepto (z)
10^-21
SI Units
Boyle's Law
states that for a fixed quantity of gas maintained at constant temperature, the volume of the gas is inversely proportional to the pressure; the product of the pressure (P) multiplied by the volume (V) remains constant if there is no change in temperature or in the number of particles inside the container; the pressure doubles when a gas is compressed to half its volume at constant temperature
PV = constant
Charles's Law
states that for a fixed quantity of gas at constant pressure, the volume of gas is directly proportional to its temperature; the ratio between the volume (V) of a gas and its temperature (T) remains constant if the pressure does not change; doubling the temperature doubles the gas's volume if the pressure does not change
V/T = constant
Avogadro's Law
states that at constant temperature and pressure, the volume of the gas is directly proportional to the moles of gas; equal values of different gases all contain the same number of particles if they all have the same pressure and temperature
Ideal Gas Law
PV = nRT
(P - pressure)
(V - volume)
(n - # of moles of gas)
(T - absolute temperature)
(R - universal gas constant - 8.314 joules per kelvin per mole)
Ways gas pressure can be doubled:
1. Gas can be squeezed into 1/2 its original volume
2. Twice as much gas can be forced into the original volume
3. Absolute temperature can be doubled
Solution
A homogeneous mixture of two or more individual substances; occurs when a substance dissolves in another; consists of solutes and solvents
Solute
substance that is dissolved
Solvent
substance that causes another substance to dissolve
Concentration of a solution
the amount of solute present in a given amount of solution
Concentrated solution
relatively large quantity of solute per unit amount of solution
Dilute solution
relatively small quantity of solute per unit amount of solution
Solubility
measure of how much solute dissolves in a given amount if solvent at a given temperature
Percent by weight
the percentage of mass of a component of a solute in a given mass of the solution
% by weight of solute = (grams solute/grams solution) ⋅ 100
Mole fraction (X)
ratio of the number of moles of a component to the total number of moles of all the components
X = (moles component/moles all components)
Molarity (M)
number of moles of solute in a liter of solution
M = (moles solute/liters solution)
Molality (m) of a solution
number of moles of solute in a kilogram of solvent
Mixture
two or more substances mixed together but not chemically combined
Homogeneous mixture
uniform throughout, like a solution
(ex: sugar and water)
Heterogeneous mixture
consists of two or more easily identifiable substances
(ex: sand and water)
As temperature increases
it's solubility increases for most solids and decreases for most gases
As pressure increases
the gases solubility increases
Saturation
the point at which a solution reaches a point where no more solute can be dissolved in it at the same temperature and pressure
If the solute-solvent attraction is stronger than the solvent-solvent attraction
the solute will dissolve readily
If the solute-solvent forces are weaker than the other forces
only a relatively small amount of the solute will dissolve
Solution forming process
1. Separation of solute molecules
2. Separation of solvent particles
3. Mixing of solvent and solute molecules so that the solute particles occupy positions that are normally taken by solvent molecules
Crystallization
process by which matter forms crystals, can occur once a solution has passed the saturation point
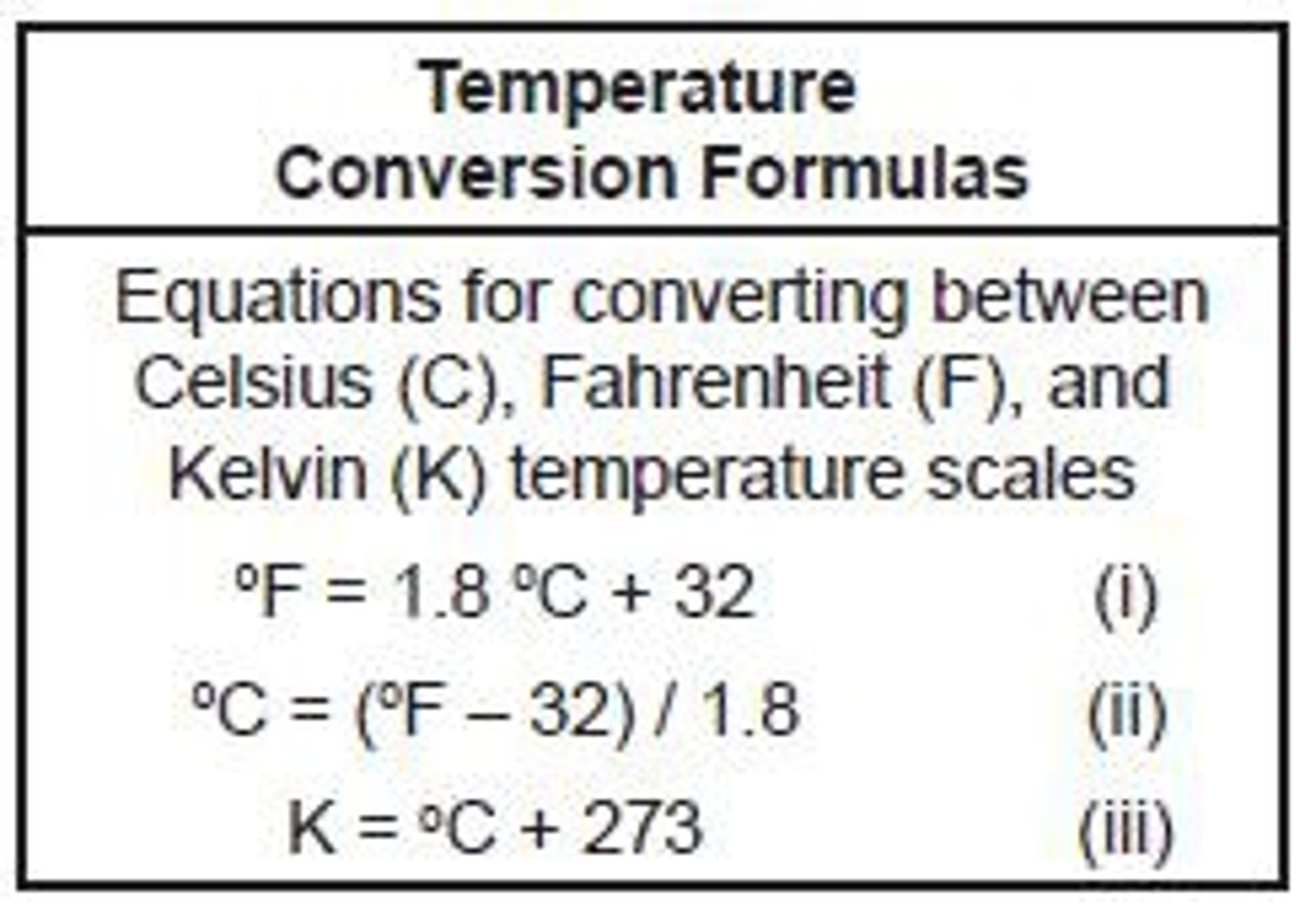
Units of temperature and conversion
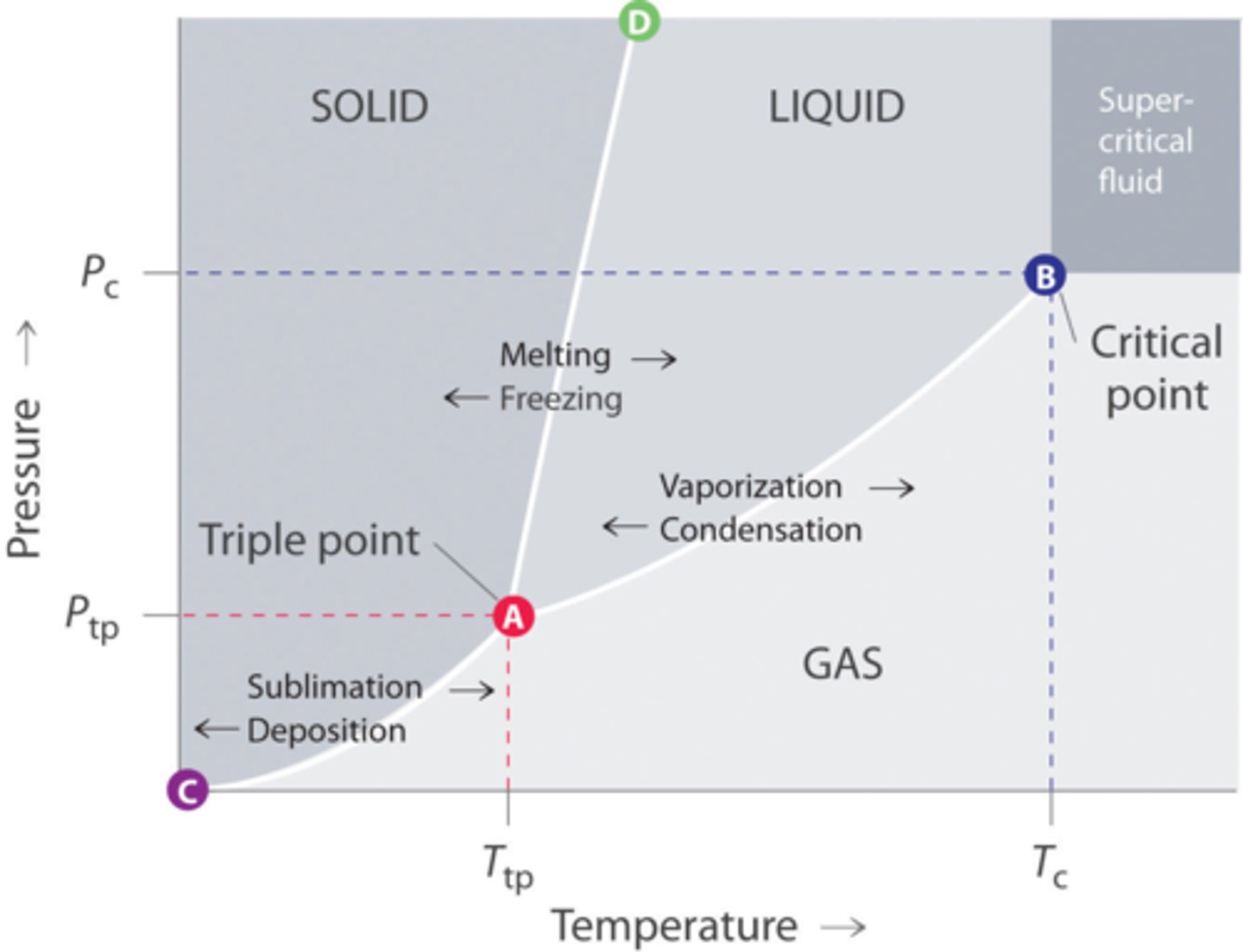
Phase changes
at certain combinations of temperature and pressure, ice changes directly to steam without becoming a liquid first
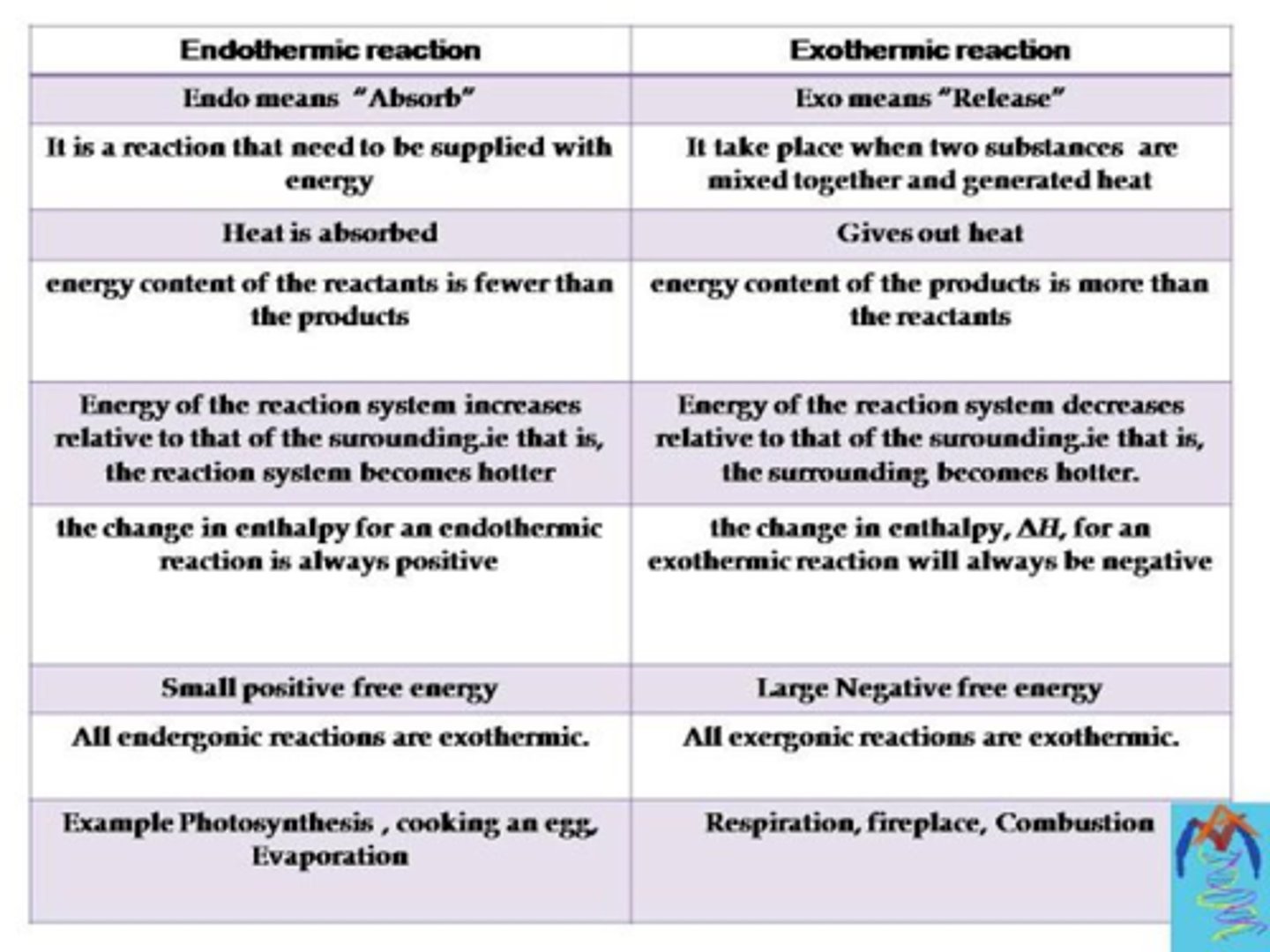
Endothermic and exothermic reactions
Endothermic: changes in which energy is absorbed
Exothermic: changes in which energy is released
1- Ions
Acetate (CH3COO)
Benzoate (C6H5COO)
Chlorate (ClO3)
Chlorite (ClO2)
Cyanide (CN)
Dihydrogen Phosphate (H2PO4)
Glutamate (C5H8NO4)
Hydrogen Carbonate/Bicarbonate (HCO3)
Hydrogen Oxalate (HOOCCOO)
Hydrogen Sulfate/Bisulfate (HSO4)
Hydrogen Sulfide/Bisulfide (HS)
Hydrogen Sulfite/Bisulfite (HSO3)
Hydroxide (OH)
Hypochlorite (CLO) or (OCl)
Nitrate (NO3)
Nitrite (NO2)
Perchlorate (CLO4)
Permanganate (MnO4)
Stearate (C12H35COO)
Thiocyanate (SCN)
2- Ions
Carbonate (CO3)
Chromate (CrO4)
Dichromate (Cr2O7)
Hydrogen Phosphate (HPO4)
Oxalate (OOCCOO)
Silicate (SiO3)
Sulfate (SO4)
Sulfite (SO3)
Tetraborate (B4O7)
Thiosulfate (S2O3)
3- Ions
Borate (BO3)
Citrate (C3H4OH(COO)3)
Phosphate (PO4)
5- Ion
Tripolyphosphate (P3O10)
1+ Ions
Ammonium (NH4)
Hydronium (H3O)
2+ Ion
Mercury/l (Hg2)
The Dalton Model
described atoms as small, spherical, indivisible particles; theory states that each element is composed of its own kind of atoms, all with the same relative weight; explained why a fixed weight of one substance always combines with a fixed weight of another substance in forming a compound
Thompson Model
described the atom as consisting of smaller subatomic particles; discovered the first subatomic particle: the electron; determined that since all atoms are electrically neutral, the atoms must contain as many positive charges as they do negatively charged electrons
Rutherford Model
stated that the atom is mostly empty space; proposed the theory that the atom consists of a nucleus with a positive charge with enough electrons rotating around it to balance the charge; these electrons were kept within the atom by the attraction of the positively charged nucleus
Bohr Model
proposed that an atom's electrons could travel only in certain successively larger orbits around the nucleus; through the outer orbits could hold more electrons than the inner ones; suggested that the electrons in the outermost orbit determined the atom's chemical properties
Quantum Mechanics
explains the structure of atoms, the way they give off light, and other related matters
Principal Quantum Number
n
Higher quantum number
higher orbital energies
Ground state
lowest energy level; where hydrogen electrons were normally
Excited state
when an electron becomes unstable due to the absorption of energy
Acids and bases
two types of chemical compounds characterized by their opposite effects in certain physical and chemical properties and their ability to neutralize each other in a chemical reaction
Acids
occur naturally and some are essential for life; many acids are poisonous and strong acids can cause severe burns
(ex: Hydrochloric Acid (HCl) produced in the stomach to aid in digestion)
Bases
have many practical uses
(ex: Magnesium Hydroxide (Mg(OH)2) is often used as an ingredient in antacids)
Arrhenius Theory
observed that all substances classified as acids contain hydrogen ions, H+, and that all substances known as bases contain the hydroxide ions, OH-
Arrhenius acid
substance whose water solution contains a high concentration of hydrogen ions
Arrhenius base
substance whose water solution contains a high concentration of hydroxide ions
Acid properties
- have a sour taste and produce a prickling or burning sensation if they come into contact with the skin
- dissolve many metals
- turn blue litmus paper red
- are neutralized by bases
Base properties
- react with an acid to decrease or neutralize its acidic properties
- also called alkalis
- turn red litmus paper blue
- feel slippery and taste bitter when dissolved in water
Bronsted-Lowry Theory
theorized that an acid-base reaction is a proton-transfer reaction in which a proton (a hydrogen ion) is transferred from the acid to the base
theorized that the strength or weakness of different acids and bases is a measure of their tendency to lose or gain protons
Bronsted acid
proton (H+) donor
Bronsted base
proton acceptor
Bronstead-Lowry reaction
B + HA < ⇌ > HB+ A-
(B = base proton receiver)
(HA = acid proton receiver)
(A- = base)
(HB+ = acid)
Conjugate acid-base pairs
when combinations such as the acid HA and the base A- result from an acid losing a proton or a base gaining one
Acid: Hydrochloric Acid (HCl)
Base: Chloride ion (Cl-)
Acid: Nitric Acid (HNO3)
Base: Nitrate ion (NO3-)
Acid: Hydrocyanic Acid (HCN)
Base: Cyanide ion (CN-)
Acid: Perchloric Acid (HClO4)
Base: Perchlorate ion (ClO4-)
Acid: Sulfuric Acid (H2SO4)
Base: Hydrogen Sulfate ion (HSO4-)
A strong acid
donates protons easily
A weak acid
clings to its protons
A strong base
has a strong attraction for protons
A weak base
has a weak attraction for protons
The stronger the acid
the weaker its conjugate base
The weaker the acid
the stronger its conjugate base
Chemical Formulae

Compounds
substances that contain more than one kind of atom
Also includes:
- Hydrogen Peroxide (H2O2)
- Glucose (C6H12O6)
- Sucrose (C12H22O11)
- Propane (C3H8)
- Octane (C8H18)
- Methanol (CH3OH)
- Ethanol (C2H5OH)
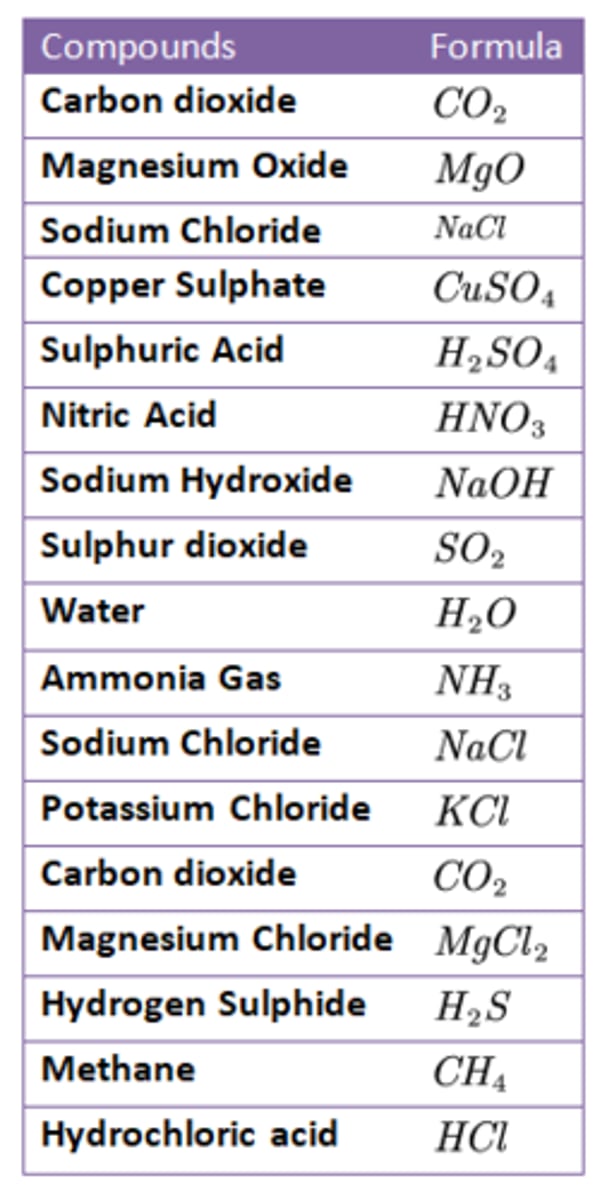
Molecular formulas
show the exact number and type of atoms combined in each molecule of a compound
(ex: Methane, CH4, contains one carbon atom and four hydrogen atoms)
Empirical formulas
show the simplest ratios of the ions--atoms or molecules that have an electric charge--present in an ionic compound
(ex: Sodium Chloride is NaCl, while Barium Fluoride is BaF2)
Ionic compounds do not form individual, discrete molecules but instead are arranged in a crystal lattice; their empirical formula must reflect an overall net charge of zero