Chemistry Ionic Bonds
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
20 Terms
What ion does Sulfur (S) make?
-2
Why do atoms form bonds with each other?
to get a complete outer shell of electrons
Which of these elements exist as single atoms because they already have a full valence shell (Octet Rule)?
Neon(Ne)
What is a cation
a positive charged ion
Which of the following describes what happens when an atom of Magnesium (Mg) bonds with an atom of Oxygen (O)?
the magnesium loses two electrons to the oxygen

Which is the correct name for the compound below?
Calcium chloride

The green box represents
a space
Which of these elements is most likely to gain electrons when forming bonds?
fluorine (F)
What are the two exceptions to the octet rule?
Hydrogen & Helium
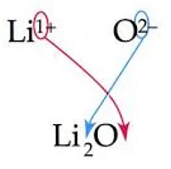
This is an example of a(n)
Ionic Bond
How many valence electrons do most atoms need to be chemically stable (Octet Rule)?
8
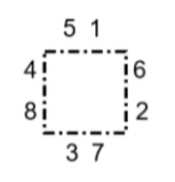
The diagram shows
the order of distributing valence electrons and spaces around elements.
What do we call an element that has a charge due to lost or gained electrons.
ION
How do you determine the number of valence electrons in an atom?
By looking at the Periodic Table and finding its group number.
What types of atoms make ionic bonds with each other?
metal and nonmetal

The difference between a Bohr Model of the atom and a Lewis Dot Diagram of the atom is
the Bohr Model shows all electrons and the Lewis Dot Diagram shows only the valence electrrons.
What is a valence electron?
An electron in outermost energy level.

The electron dot diagram for aluminum is shown. How does an atom of Aluminum satisfy the Octet Rule?
loses three electrons to a nonmetal
What is an anion?
a negatively charged ion
What ion does Strontium (Sr) form?
+2