Battery Technology (ACE)
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
Primary Batteries
Galvanic cells that produce electricity from sealed-in chemicals
Cannot be recharged; once equilibrium is reached, they are discharged
Example: dry cell, lithium copper sulfide cell
Requirements:
Economically and environmentally benign
Provide a constant voltage and a long discharge duration
High energy density and longer shelf life
Compact, lightweight and made from easily available materials
Secondary Batteries
Rechargeable cells that can be used through multiple charging and discharging cycles
External electricity reverses the spontaneous cell reaction during charging.
Ex: Nickel cadmium cell, lead acid battery, lithium ion battery
Requirements:
High power-to-weight ratio
Provide high voltage
Long cycle life and shelf life
Short recharge time
Galvanic Cells
Convert chemical to electrical energy
Produces electricity as a result of the spontaneous redox reaction occurring inside it
Anode (-):
Oxidation occurs
Electrons are produced
Desired characteristics: Low reduction/redox potential, high specific capacity, reversibility, and good conductivity.
Cathode (+):
Reduction occurs
Electrons are consumed
Desired characteristics: High reduction potential, high specific capacity, reversibility, and stability.
Galvanic vs Electrolytic Cells
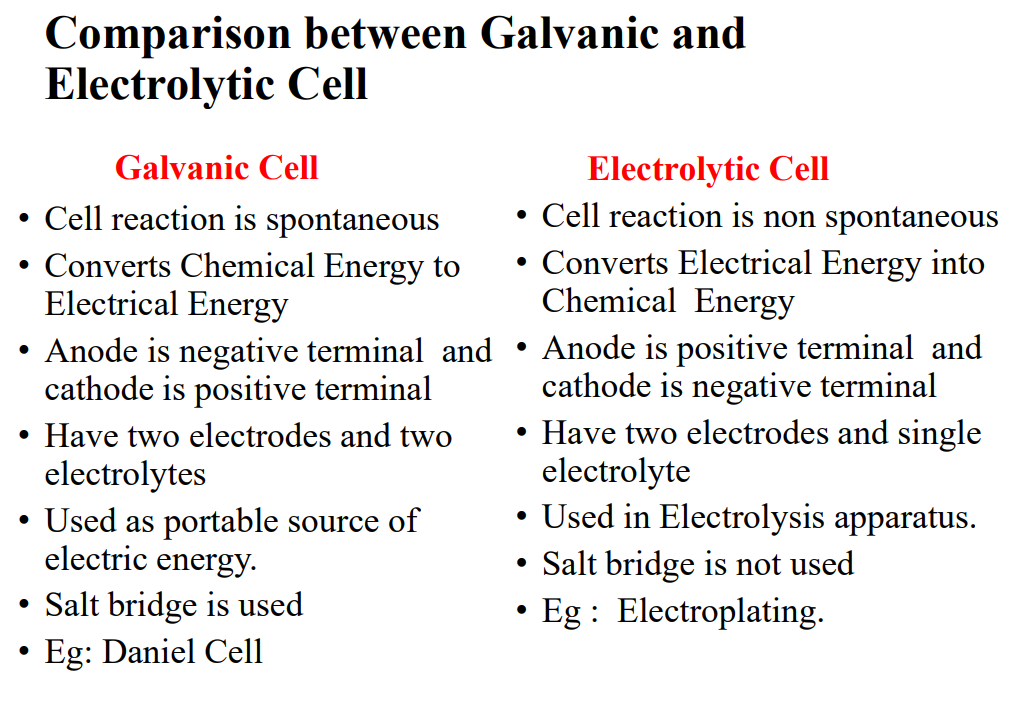
Daniel Cell
The cell consists of a zinc electrode in a zinc sulfate solution and a copper electrode in a copper sulfate solution
Anode: Zn → Zn2+ +2e-
Cathode: Cu2+ + 2e- → Cu
Cell reaction: Zn + Cu2+ → Zn2+ + Cu
Key Battery Performance Metrics
Current
Capacity
C = (m x n x F)/M
m → mass of active material
M → molar mass
n → number of electrons transferred
Energy efficiency
% Energy efficiency = (Energy released on discharge / Energy required for charging) × 100
Cycle life
Number of charge-discharge cycles that can be achieved before failure occurs
Shelf life
Energy Density
Power density
Traditional Liquid Electrolyte
Low vicosity, high energy density
High discharge, charge rate
Operational temperature: -40 to 60
Low flammability
Polymetric Electrolytes
Solid or gel form
Solid: High flexibility, energy density, safety and stability
Gel: High ionic conductivity, multifunctional, chemically stable
Advantages of Solid Polymeric Electrolytes:
No leakage.
Non-flammable.
Non-volatile.
Thermal and mechanical stability.
Easy fabrication.
High achievable power density and cyclability.
Lead Acid Battery/Storage Battery
Anode: spongy lead (Pb)
Cathode: lead dioxide (PbO2)
Electrolyte: sulfuric acid solution (H2SO4)
Anode reaction:
Pb → Pb2+ + 2e-
Pb2+ + SO42- → PbSO4
Overall: Pb + SO42- → PbSO4 + 2e-
Cathode reaction:
PbO2 + 4H+ + 2e- → Pb2+ + 2H2O
Pb2+ + SO42- → PbSO4
Overall: PbO2 + 4H+ + 2e- + SO42- → PbSO4 + 2H2O
Overall reaction: Pb + PbO2 + 4H+ + 2SO42- → 2PbSO4 + 2H2O
Overcharging of Lead Acid Batteries
If charging is pushed beyond full capacity, electrolysis of water occurs.
Anode: 2H2O → O2 + 4H+ + 4e-
Cathode: 2H+ + 2e- → H2
Overall: 2H2O → O2 + 2H2
Consequences:
Lowers acid level → damages electrode grids.
Gas pressure may build up → risk of explosion.
Older batteries require topping up with water.
Solution:
Maintenance-free batteries
Pb-Ca alloy anode reduces water electrolysis
Catalysts (ceria + platinum) recombine gases into water; no maintenance is needed
Sealed design prevents leakage
Applications of Lead Acid
Automotive Batteries: Starting, Lighting, Ignition (SLI) for cars and trucks
Industrial Batteries: Motive and standby power for heavy-duty applications
Consumer Batteries: Emergency lighting, security systems, power tools, UPS
Advantages of Lead Acid
High Efficiency: Voltage efficiency ~80%
Longevity: 300–1500 recharge cycles (up to 2000 for sealed batteries)
Quick Recharging: Approximately 2–8 hours
Low Self-Discharge: Maintains charge when not in use
High Current Capability: 12 V car batteries can deliver over 10 A
Disadvantages of Lead Acid
Sulfation: Formation of large PbSO₄ crystals if left partially charged
Weight: Low energy-to-weight ratio (~35 Wh/kg)
Concentration Dependent: Cell potential drops as sulfuric acid is consumed
Temperature Sensitivity: Reduced efficiency at lower temperatures
Overcharging Risks: Potential electrode damage and explosion
Toxicity: Environmental and health concerns with lead
Corrosion: Lead grid corrosion at the lead dioxide electrode
Nickel-Metal Hydride (NiMH) battery
Anode: Hydrogen-absorbed alloys
Cathode: Nickel hydroxide
Electrolyte: Alkaline, mainly potassium hydroxide
Anode reaction:
MH + OH- → M + H2O + e-
Cathode reaction:
NiOOH + H2O + e- → Ni(OH)2
Overall Reaction:"
MH + NiOOH → M + Ni(OH)2
They have about twice the energy density of Ni-Cd batteries but a similar operating voltage
Advantages of NiMH battery
High Energy Density: Surpasses Ni-Cd batteries, with capacities ranging from 1000mAh to 3000mAh or higher
Long Cycle Life: Hundreds to thousands of recharge cycles
Environmentally Friendly: Fewer harmful materials, no toxic cadmium
Safety: Stable, lower risk of thermal runaway or fire hazards
Cost-Effective: Long-term economic power solution with fewer replacements needed
Disadvantages of NiMH battery
Self-Discharge: Loses 1–5% charge per day when idle
Memory Effect: Potential issues if not fully discharged before recharging
Temperature Sensitivity: Performance affected by extreme temperatures
Charging: Slower charging rates, limited fast charging capability
Voltage Output: Lower compared to newer battery chemistries
Applications of NiMH battery
Consumer electronics
Power tools
Hybrid Vehicles
Emergency lighting and backup power
Flashlights and portable devices
Electric bicycles and scooters
Renewable energy storage
👉 ""Cats Play Violins Loudly For Big Rewards.""

Lithium Ion Batteries
Anode: Lithium-carbide type intercalate (LixC6)
Li (C) → Li+ + C + e-
Cathode: Transition metal oxide MO2 of variable oxidation state
Li+ + CoO2 + e- → LiCoO2
Overall Cell Reaction: Li (C) + CoO2 → LiCoO2 + C
The electrolyte is usually inert (polar) dry ether or carbonates(diethyl carbonate or propylene carbonate)
Applications of Lithium Ion Batteries
Used in applications where one or more of the advantages(size, weight or energy) outweigh the additional cost, such as mobile telephones and mobile computing devices.
Used when the battery design matters in a particular application, as different designs are possible (Cylindrical,jelly-roll design, flat rectangular).
Used in current-generation laptops, cellular phones, videorecorders, portable CD players, televisions and implantable medical devices.
Mobile telephones/cellular phones → (has a screen) → laptop, televisions → (video related) → videorecorders, portable CD players
Advantages of Lithium Ion Batteries
1. Designed to overcome the safety problems associated with the highly reactive properties of Lithium metal.
2. Long cycle life (400-1200 cycles).
3. Smaller, lighter and provide greater energy density than either nickel-cadmium or nickel-metal-hydride batteries
4. Can be operated in a wide temperature range and can be recharged before they are fully charged.
5. Typically designed to be recharged in the device rather than in an external charger.
6. The average voltage of a Li-ion battery is equivalent to three Ni-Cd cells.
7. A typical Li-ion battery can store 150 watt-hours of electricity in 1kilogram of battery, as compared to lead acid batteries, which can store only 25 watt-hours of electricity in one kilogram.
Disadvantages of Lithium Ion Batteries
Poor charge retention
Self-discharge rate is about 10% per month
High cost
Traditional Li-ion batteries rely on liquid electrolytes, which are flammable and can pose a fire risk if the battery ruptures or overheats
Safety Concerns with Lithium-Ion Batteries
Damage, puncture or malfunction can lead to dangerous heating, pressure buildup and thermal runaway
Over time, batteries degrade, becoming more prone to overheating and thermal runaway
Safety Measures and Environmental Impact of Battery Production
Battery Management System: Monitors and controls charging/discharging to prevent hazards.
Thermal Management Systems: Utilize heat sinks and fans to prevent thermal runaway.
Quality Control and Testing: Ensures detection and correction of cell flaws before market entry
Safety Standards and Regulations: Established by authorities to ensure safe battery use.
Sodium-Ion Batteries
Lithium (Li): Limited supply, energy-intensive extraction, costly and geographically restricted.
Sodium (Na): Abundant (6th most in Earth’s crust, seawater, salt), simpler extraction, cheaper and more stable supply → promising for large-scale storage.
Similarities
Both are alkali metals (Group 1), lose one valence electron to form Li⁺ / Na⁺, making them good for batteries.
Both work by ion flow between electrodes during charge/discharge.
Differences
Ionic size: Li⁺ (0.76 Å) < Na⁺ (1.00 Å).
Larger Na⁺ ions struggle to fit into electrode materials designed for Li⁺ → lower efficiency.
Challenges & Advances in Na-ion
Electrode design: Need new materials (e.g., Prussian blue analogues, layered sodium vanadates) to handle bigger Na⁺ ions.
Performance: Current Na-ion batteries have lower energy & power density than Li-ion.
Research: Focused on optimising electrodes & electrolytes to improve efficiency.
Emerging Battery Technologies
Solid State Batteries
Solid electrolyte
High energy density and enhanced safety
Ability to revolutionize EV sector
Lithium-Sulfur Batteries
Higher energy density than lithium ion
Sulfur is more abundant and affordable
Challenges: low electric conductivity of sulfur and polysulfide dissolution
Lithium-Air Batteries
Extremely high theoretical energy density
Lithium metal anode, oxygen from air cathode
Challenges: cycle life and lithium stability
Flow Batteries
Liquid electrolytes in external tanks
Electrolytes contain active materials that undergo oxidation and reduction reactions during charge and discharge cycles.
Scalable, flexible capacity
Research on better energy density and cost
Metal Air Batteries
Metal (Zn or Al) anode, atmospheric oxygen cathode
High theoretical energy densities, potentially low cost
Challenges: Reversibility, efficiency, and cycle life
Fuel Cells
A fuel cell is a galvanic cell of a special type in which chemical energy contained in a fuel–oxidant system is converted directly into electrical energy
The reactants (i.e. fuel + oxidant) are constantly supplied from outside and the products are removed at the same rate as they are formed.
Advantages of Fuel Cells
High efficiency: 70–75% vs. only 35–40% for coal-based thermal plants.
Eco-friendly: No noise, chemical, or thermal pollution.
Decentralized use: Can be installed near point of use , reducing ~30% transmission losses.
Continuous power: Provides steady electricity as long as fresh reactants is supplied.
Methanol Fuel Cell
Electrolyte: Sulphuric acid
Electrodes: Typical gas diffusion electrodes, made up of porous C coated with Pt catalyst
Fuel: Methanol Oxidant
Air Catalyst: Platinum
Anode reaction:
CH3OH + H2O → CO2 + 6H+ + 6e-
Cathode reaction:
3/2 O2 + 6H+ + 6e- → 3H2O
Net reaction:
CH3OH + 3/2 O2 → CO2 + 2H2O
Proton Exchange Membrane Fuel Cell
Electrolyte: Ion exchange polymeric membranes.
Electrodes: Typical gas diffusion electrodes, made up of porous C coated with Pt catalyst.
Fuel: Hydrogen
Oxidant: Air
Catalyst: Platinum
Operate at relatively low temperatures, around 80 °C → Quick start-up, less wear, better durability.
Efficiency of 60 % → Power output of 50-250kW
Anode Reaction:
H2 → 2H+ + 2e-
Cathode Reaction:
½ O2 + 2H+ + 2e- → H2O(l)
Overall Reaction:
H2 + ½ O2 → H2O
Challenges:
Require expensive platinum catalyst.
Platinum is highly sensitive to CO poisoning, so extra processing is needed to clean fuel → higher cost.
Role of Proton conducting membranes:
Functions: Acts as electrolyte (ionic conduction) and separator (keeps reactant gases apart).
Proper proton & water transport is critical.
Too dry → low proton conductivity.
Too wet → electrolyte flooding.
Advantages:
No liquid electrolyte → simpler flow control than alkaline/phosphoric acid cells.
CO₂ tolerant.
Breakthrough: DuPont’s Nafion (perfluorinated ionomer) membranes (since 1966).
Durable (>60,000 hours at 353 K).
Advantages and Disadvantages of PEMFC
Used in transportation: passenger vehicles such as cars and buses
Advantages:
Solid electrolyte provides excellent resistance to gas crossover.
Low operating temperature allows rapid start-up
Capable of high current densities
Disadvantages:
Dehydration of the membrane reduces proton conductivity, and excess water can lead to flooding of the electrolyte. Both the conditions leading to poor performance
High cost
Sensitive to poisoning by trace levels of contaminants, including CO, sulfur species and ammonia.
Environmental Impact of Battery Production
Resource Extraction: Mining for battery materials can lead to ecological damage.
Energy Intensity: High energy consumption in production; renewable energy can reduce impact.
Chemical Pollution: Handling and disposal of hazardous chemicals must be managed safely.
Water Usage: Significant water use in production; conservation and recycling are key
End-of-Life Management
Crucial Disposal Management: Essential to minimize environmental harm.
Recycling Infrastructure: Development needed to prevent pollution.
Material Reuse: Advocacy for reusing battery materials to reduce impact.
Remember as RRRReduce Environment Harm
Recycling Infrastructure
Reuse Material
Environmental Considerations
Vital Role of Batteries: Key in renewable energy and electrification transition.
Mitigation of Environmental Impact: Necessary at all life stages of batteries.
Sustainable Practices: Implementation critical for environmental protection.
Technical Advancements: Adoption can address manufacturing concerns.
Regulatory Frameworks: Establishment helps ensure environmental safety.