MCAT General Chemistry - Electrochemistry
1/39
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
Electrochemical cells
contained systems in which Oxidation–Reduction reactions occur
electrodes
locations in an electrochemical cell where oxidation and reduction take place
anode
electrode where oxidation occurs; electrons origin (negative, negative reduction potential) in galvanic cell and anion attractor (positive, positive reduction potential) in electrolytic cell
cathode
electrode where reduction occurs; electrons attractor (positive, positive reduction potential) in galvanic cell and cation attractor (negative. negative reduction potential) in electrolytic cell
electromotive force (emf)
corresponds to the voltage or electrical potential difference of the cell
positive electromotive force
cell is able to release energy; (ΔG < 0); spontaneous
negative electromotive force
cell must absorb energy; (ΔG > 0); nonspontaneous
current in electrochemical cells
inverse of movement of electrons from anode to cathode; direction of flow of a positive charge
galvanic / voltaic cells
nonrechargeable spontaneous batteries; two electrodes of distinct chemical identity are placed in separate compartments connected to each other by a conductive material
ΔG < 0
Ecell > 0
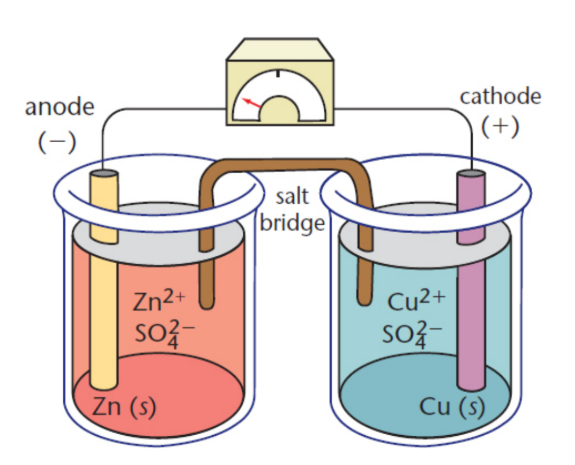
half-cells
one of the two distinct electrodes in a galvanic cell
electrolyte
aqueous ion solution composed of cations and anions
Daniell cell
galvanic cell where zinc is the anode and copper is the cathode; each electrode is bathed in an electrolyte solution containing its cation and sulfate
salt bridge
inert salt that connects the two solutions in a galvanic cell; dissipates charge gradient by permitting the exchange of cations and anions; contains ions that will not react with the electrodes or with the ions in solution
plating / galvanization
precipitation process onto the cathode
cell diagram
shorthand notation representing the reactions in an electrochemical cell
anode | anode solution (concentration) || cathode solution (concentration) | cathode
single vertical line ( | ) indicates a phase boundary.
double vertical line ( || ) indicates the presence of a salt bridge or
some other type of barrier.
ex. Zn (s) | Zn2+ (1 M) || Cu2+ (1 M) | Cu (s)
electrolytic cells
house nonspontaneous reactions that require the input of energy to proceed
molten NaCl is decomposed into Cl2(g) and Na(l)
ΔG > 0
Ecell < 0
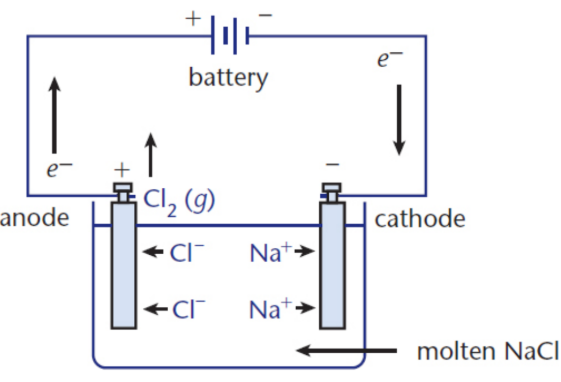
electrolysis
Oxidation–Reduction reaction driven by an external voltage source in which chemical compounds are decomposed
Faraday equation of Oxidation–Reduction
the amount of chemical change induced in an electrolytic cell is directly proportional to the number of moles of electrons that are exchanged
Mn+ + n e– → M (s)
Faraday constant, (F)
equivalent to the amount of charge contained in one mole of electrons
1 F = 96,485 C
electrodeposition equation
helps determine the number of moles of element being deposited on a plate; also used to determine the amount of gas liberated during electrolysis
mol M = It/nF
where mol M is the amount of metal ion being deposited at a specific electrode, I is current, t is time, n is the number of electron equivalents for a specific metal ion, and F is the Faraday constant
concentration cell
special type of galvanic cell where the electrodes are chemically identical; current is generated as a function of a concentration gradient established between the two solutions surrounding the electrodes; drives the movement of electrons in the direction that results in equilibration of the ion when the current will stop
emf → 0
Nernst equation
calculates the voltage as a function of concentrations in a concentration cell
Ecell = E∘cell − (RT/nF)lnQ = E∘cell - 0.0592/n log Q
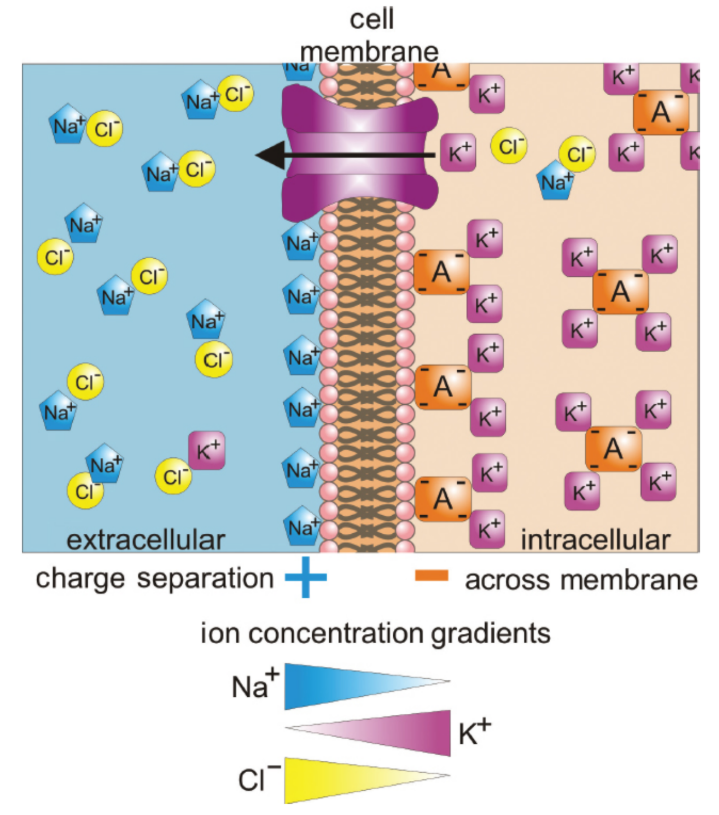
resting membrane potential (Vm)
Sodium and potassium cations and chlorine anions are exchanged as needed to produce an electrical potential across a cellular membrane; Disturbances may stimulate the firing of an action potential
rechargeable cell / battery
function as both a galvanic and electrolytic cell
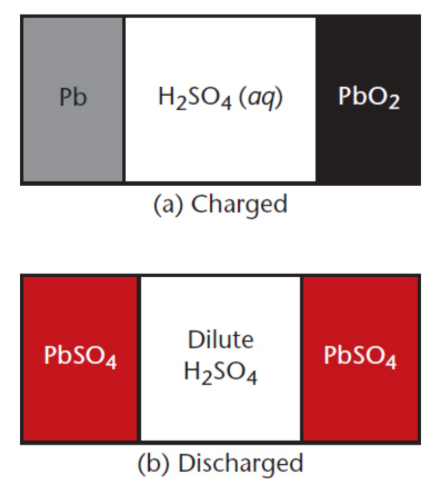
lead–acid battery / lead storage battery
rechargeable battery; low energy density
fully charged = Pb anode and porous PbO2 cathode, connected by a conductive material (concentrated 4 M H2SO4)
discharging = electrodes plate with lead sulfate (PbSO4) and dilute the acid electrolyte
fully discharged = two PbSO4 electroplated lead electrodes with a dilute concentration of H2SO4
charging = reverses the electroplating process and concentrates the acid solution
discharge
lose electric potential in a battery by changing the composition of electrodes; may be irreversible or reversible; galvanic
charging
foster electric potential in a battery by restoring the electrodes; electrolytic
Energy density
measure of a battery’s ability to produce power as a function of its weight
Nickel–cadmium batteries
rechargeable cells; two half-cells made of solid cadmium (the anode) and nickel(III) oxide-hydroxide (the cathode) connected by a conductive material, typically potassium hydroxide (KOH); higher energy density
ex. AA and AAA cells
Surge currents
periods of large current (amperage) early in the discharge cycle; preferable in appliances such as remote controls that demand rapid responses
nickel–metal hydride (NiMH) batteries
more efficient, more energy density, more cost effective, less toxic modern alternative to Ni-Cd batteries
Isoelectric focusing
technique used to separate amino acids or polypeptides based on their isoelectric points (pI); positively charged / protonated amino acids migrate toward the cathode; negatively charged / deprotonted amino acids migrate toward the anode
standard hydrogen electrode (SHE),
relative standard of reduction potential; 0 V by convention
reduction potential
the tendency of a species to gain electrons and to be reduced; more positive the potential, the greater the tendency to be reduced
Standard reduction potential (E°red)
measured under standard conditions: 25°C (298 K), 1 atm pressure, and 1 M concentrations; predict the direction of electron flow
oxidation potential
reduction half-reaction and the sign of the reduction potential are reversed
standard electromotive force (emf or E°cell)
difference in potential (voltage) between two half-cells under standard conditions; do NOT multiply them by the number of moles oxidized or reduced
E°cell = E°red,cathode − E°red,anode
Gibbs Free Energy in an electrochemical cell
change in the amount of energy available in a chemical system to do work
ΔG° = –nFE°cell
where ΔG° is the standard change in free energy, n is the number of moles of electrons exchanged, F is the Faraday constant, and E°cell is the standard emf of the cell
ΔG° and E°cell will always have opposite signs
Gibbs Free Energy in equilibrium
ΔG° = –RT ln Keq
where R is the ideal gas constant, T is the absolute temperature, and Keq is the equilibrium constant for the reaction
equilibrium constants less than 1 (favors the reactants), the E°cell will be negative
equilibrium constant greater than 1 (favors the products), the E°cell will be positive
Gibbs Free Energy in nonstandard conditions
ΔG = ΔG° + RT ln Q
where ΔG is the free energy change under nonstandard conditions, ΔG° is the free energy change under standard conditions, R is the ideal gas constant, T is the temperature, and Q is the reaction quotient