Reaction of ethanal with Acidified KMnO4, Fehling's reagent, Tollen's reagent
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
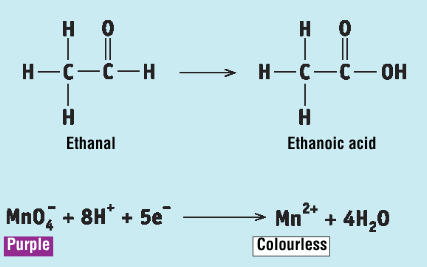
Key steps + observations in the procedure for the reaction of ethanal and propanone with acidified KMnO4
in a test tube some potassium permanganate is acidified with dilute sulfuric acid
ethanal is added, and the test tube is placed in a beaker of warm water
the ethanal is oxidised to ethanoic acid, and the acidified potassium permanganate changes from purple to colourless as it has been reduced
when the experiment is repeated with propanone, the potassium permanganate solution does not change colour as ketones are not oxidised by potassium permanganate
Write the 2 half-reactions that took place in the reaction of ethanal with acidified KMnO4
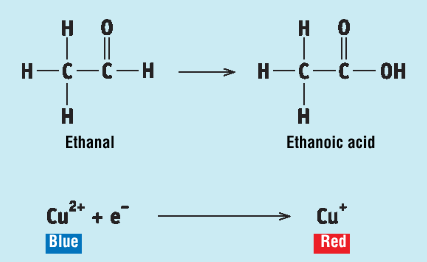
Key steps + observations in the procedure for the reaction of ethanal and propanone with Fehling’s reagent
Fehling’s reagent is made up by mixing equal quantities of Fehling’s A and Fehling’s B, forming a clear royal blue solution
in a test tube containing Fehling’s reagent, some ethanal is added
the test tube is placed in a beaker of warm water, and the ethanal is oxidised to ethanoic acid
a brick-red precipitate is observed as the Cu2+ ions (blue) in the Fehling’s reagent are reduced to Cu+ ions (red) by the ethanal
when the experiment is repeated with propanone, no red precipitate is observed when the Fehling’as reagent and propanone are heated together, as ketones arenot oxidised by Fehling’s reagent since it is a weak oxidising agent
What is Fehling’s A and Fehling’s B?
Fehling’s A - a blue solution of copper sulfate in water
Fehling’s B - a colourless solution of potassium sodium tartrate (Rochelle salt) and sodium hydroxide in water
Write the 2 half-reactions that took place in the reaction of ethanal with Fehling’s reagent
Key steps + observations in the procedure for the reaction of ethanal and propanone with ammoniacal silver nitrate (silver mirror test)
Tollens’ reagent is freshly made by mixing silver nitrate solution, dilute sodium hydroxide and dilute ammonia solution in appropriate quantities
in a clean, dry test tube, some Tollens’ reagent and ethanal are added
the test tube is placed in a beaker of warm water, and it is observed that a silver mirror is formed on the inside of the test tube
when this experiment is repreated with propanone, no change is observed when the Tollens’ reagent and propanone are heated together, as Ketones are not oxidised by Tollens’ reagent since it is a weak oxidising agent
What is Tollens’ reagent?
silver oxide dissolves in ammonia solution to form a sollution called ammoniacal silver nitrate
Write the 2 half-reactions that took place in the reaction of ethanal with Tollen’s reagent

Why must Tollen’s reagent be freshly made-up?
If stored after being made up, it is likely that explosive products will be formed
Since Ketones are not easily oxidised, what can be used to oxidise them?
very strong oxidising agents such as nitric acid must be used, as they have the effect of bringing about oxidation in a destructive way by breaking carbon-carbon bonds causing the ketones to be broken up
Describe the appearance of ethanal
colourless liquid
What is the name of the brick-red compound which precipitates when Fehling’s reagent is reduced?
Cu2O - Copper (I) oxide
Why are reactions involving ethanal slower and must be heated?