shapes and IMF's
0.0(0)
Card Sorting
1/6
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
1
New cards
what do electron pairs around an atom determine
its shape
2
New cards
what do wedges show
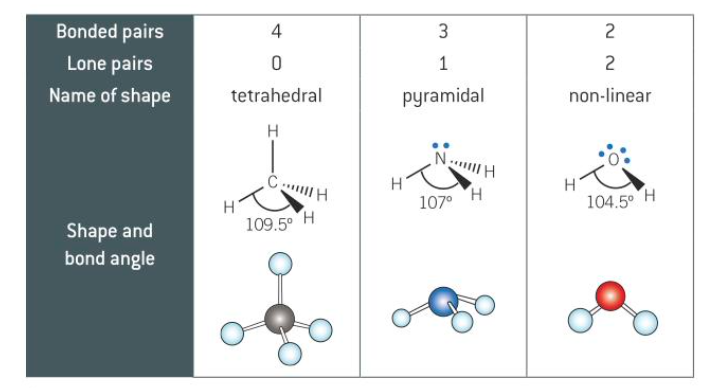
3
New cards
bonded pairs and lone pairs
lone pairs lie closer to the atom than bonded pairs so occupies more space which causes the lone pair to repel more
4
New cards
how much more does a lone pair repel
2.5 degrees
5
New cards
what do electrons do to each other
repel as far as possible
6
New cards
shape, name and bond angle of 2,3,4,6
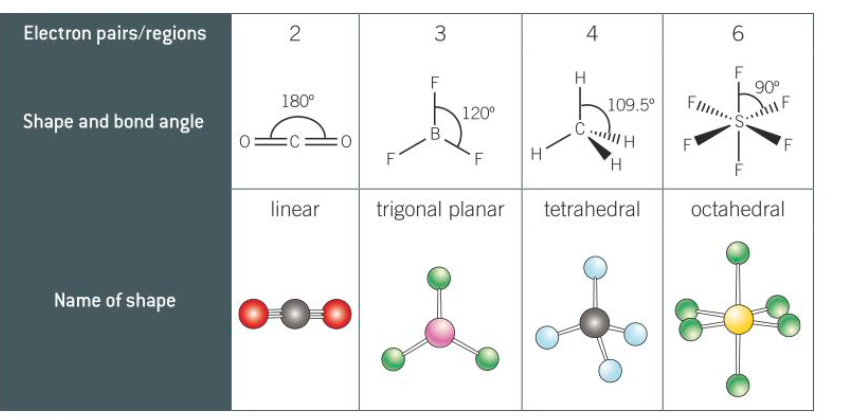
7
New cards
shape, name, bond angle of 2,3,4 with lone pairs