Effect of Osmosis on Plant tissues
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
Describe the experiment
1)Peel the tomato beacsue the potato skin can affect osmosis
2)Use a cork borer to produce three cylinders of potato. Using a cork borer makes all of the cylinders the same diameter
3)Use a scalpel to trim the cylinders to the same length(3cm)
4)Measure the length of the ruler and the mass of each cylinder using a balance
5)Place each cylinder into a test tube. First tube add 10cm3 of 0.5 molar sugar solution.Add 10cm3 of 0.25 molar sugar solution to the second test tube and 10cm3 of distilled water in third test tube
6)Leave the cylinders overnight to allow osmosis to take place
7)Remove the potato cylinders and gently roll them on paper towel to remove any surface moisture
8)Measure length and mass of cylinders again
9)Calculate percentage change in mass and length. Then plot a few graphs
Why do we use distilled water instead of tap water
As it contains not dissolved substances which can affect rate of osmosis
What is the dependent variable
Mass and length of the potato cylinders
What is the independent variable
The concentration of the sugar solution
What is the control variable
Volume of solution,temperature,time,type of sugar used
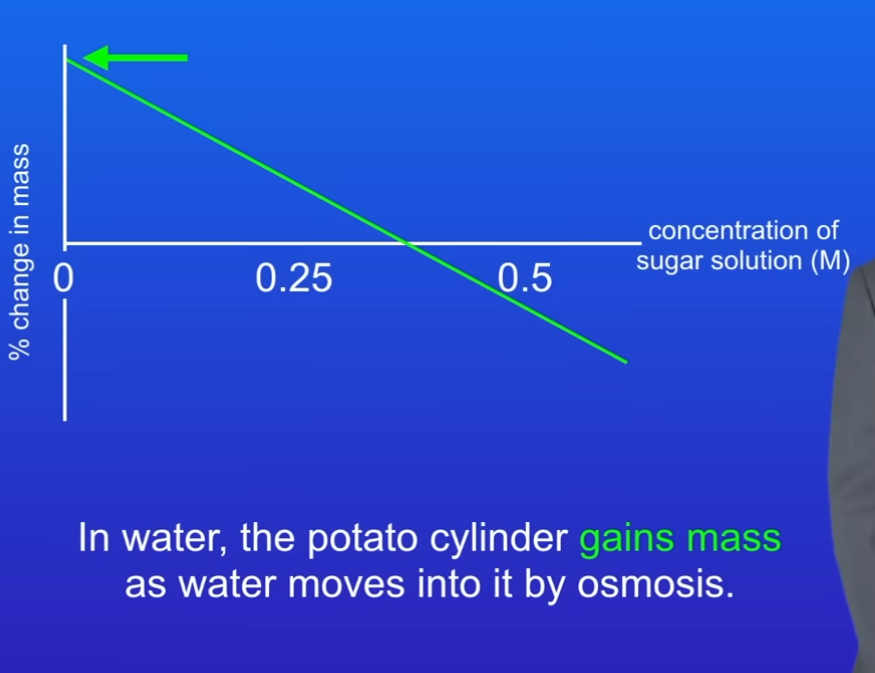
Describe results with table
-In water the potato cylinder gains mass as water moves in by osmosis
-In concentrated sugar solution, the cylinder loses mass as water moves out by osmosis
-Where the line crosses the x acid there is no change in mass. This is because concentration outside the cell is the same inside so no osmosis takes place
What are some errors and how can we fix them
-Sometimes potato cylinders were not fully dried so excess water give a higher mass or if water evaporated from the beaker, the concentration of sugar solution changes.
-You can reduce errors by repeating the experiment and calculating a mean percentage change at each concentration