Wave particle duality- Model Answers
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
Define the intensity of light in the wave model of light.
Intensity is power/area.
Intensity is proportional to the amplitude of the wave squared - this is related to the energy of the wave.
Define photon
Discrete packet of electromagnetic energy.
Describe what is meant by the wave particle duality of light.
Light exhibits wave behaviour (e.g. diffraction, interference, superposition, polarisation).
Light exhibits particle behaviour (e.g. absorption and emission line spectra, photoelectric effect).
So light exhibits both wave and particle behaviour: it exhibits wave particle duality.
Define work function
Minimum energy required for electron to be released from the surface of a metal.
Define threshold frequency
Minimum photon frequency required for electron to be released from the surface of a metal (by absorbing the photon).
Explain how the equation hf = Φ + KE max is a conservation of energy equation.
Hf is the photon energy
When the electron absorbs the photon, the photon energy is transferred to the electron
The electron leaves the surface of the metal using a minimum amount of energy called the work function Φ.
The remaining energy is transferred into the kinetic energy of the electron.
Which will have a maximum value of KEmax when the electron has only used the work function to leave the surface.
Explain why the kinetic energy of the electron in the equation hf = Φ + KE max is the maximum kinetic energy.
The work function is the minimum energy required for electron to be released.
Thus in the equation hf = work function + KE max, the kinetic energy is the maximum possible kinetic energy.
Most electrons will have less kinetic energy than this, as they require more energy in order to leave the surface of the metal (due to needing more than the minimum energy, to overcome the electrostatic forces of attraction).
Plot a graph of maximum kinetic energy of released electron against frequency of photon.
(y intercept is negative work function)
hf = Φ + KE
Graph of KE against f
KE = hf - Φ
y = mx - c
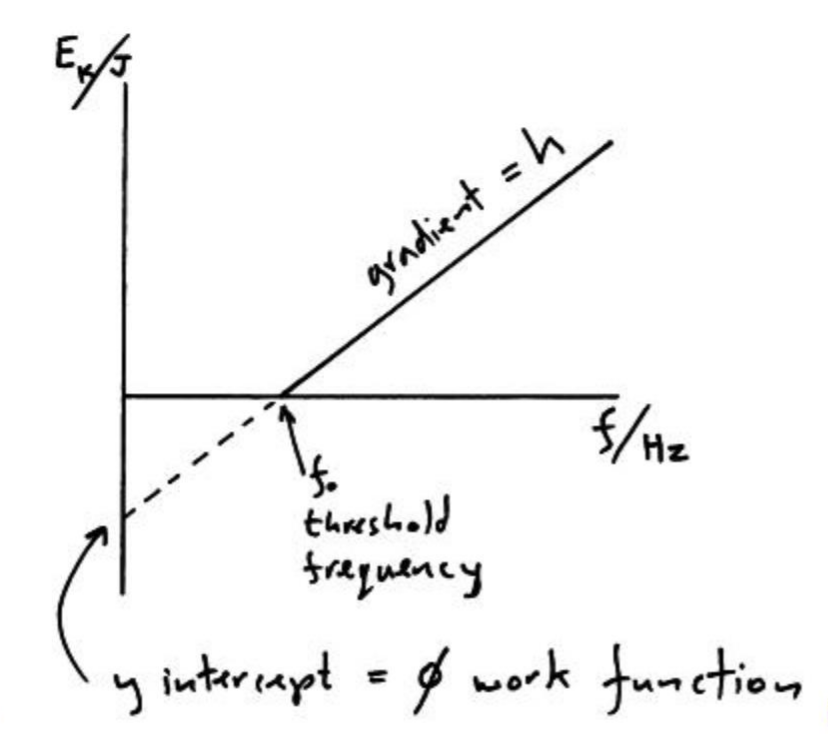
Derive the equation relating threshold frequency and work function.
The threshold frequency is the photon frequency that would cause electrons to just leave the metal surface but have no kinetic energy on escaping.
The equation hf = Φ + KE max becomes
hf0 = Φ where f0 is the work function
so f0 = Φ/h
Describe the photoelectric effect observations and conclusions.
Observation : Electrons are released instantaneously from the matal surface. Expected by wave model: The energy would take time to be transferred from the light to the electron. Particle model: One electron absorbs one photon, absorbing its energy as a packet.
Observation : The kinetic energy of electrons is dependent on frequency of incident light. Expected by wave model: The kinetic energy of the electron should be dependent on the intensity of the light, as intensity is related to the enegy of the light in the waves model. Particle model: The energy of a photon is equal to hf.
Observation : Only light above a certain threshold frequency value will cause emission of electrons. Expected by wave model: Any frequency of light should release electrons as long as the intensity is high enough (energy in the wave model is dependent on intensity). Particle model: The minimum photon energy to release an electron from the metal surface is equal to the work function, Φ of the metal. This means there is a minimum threshold frequence f = Φ / h required to release the electron.
Observation : Increasing intensity of light increases the number of electrons emitted per second. Expected by wave model: Increasing intensity should increase the energy of emitted electrons. Particle model: Intensity = power/area = rate of energy transfer per second/area.
Intensity = (number of photons x energy of one photon) / (time x area)
I = Nhf / tA
So the intensity is directly proportional to the number of photons per second.
As one photon is absorbed by one electron, this is also proportional to the number of electrons relased per second.
Explain how the photoelectric effect indicates that light is a particle.
In the wave model, energy would be absorbed over time and so it would take time for the electrons to be released. - this is not observed.
Instead, it is observed in the photoelectric effect that electrons are emitted instantaneously from the metal.
This indicates that one photon is absorbed by one electron.
In the wave model, energy of the wave is proportional to intensity, so in the wave model, the kinetic energy of the electrons should depend on intensity - this is not observed.
Instead, it is observed that the kinetic energy of the electron is only dependent on the frequency of the photon, not intensity.
So the energy of the photon, E = hf is proportional to frequency.
It is also observed that as intensity increases, the number of electrons leaving the metal surface per second increases.
This is because more photons are absorbed each second as intensity increases.
In the wave model, high intensity light of any frequency should emit electrons - this is not obserbed.
Instead, it is observed that only EM radiation above a certain threshold frequency will release electrons.
Electrons are only released from the metal surface when energy of photon is larger than work function of the metal.
Define the intensity of light in the particle model of light, deriving its equation and stating what it is proportional to.
Intensity = power / area = rate of energy transfer per second / area
Intensity = (Number of photons x energy of one photon) / (time x area)
I = Nhf / tA
So the intensity is directly proportional to the number of photons per second.
State the effect on the maximum kinetic energy of photoelectrons emitted when the intensity of light increases.
No effect (intensity does not effect KE).
State the effect on the number of photoelectrons emitted per second, when the intensity of light increases.
The number of photoelectrons emitted per second increases, in proportion with the intensity increase.
Draw a circuit diagram that we could use to determine the stopping potential.
(SEE PHOTO)
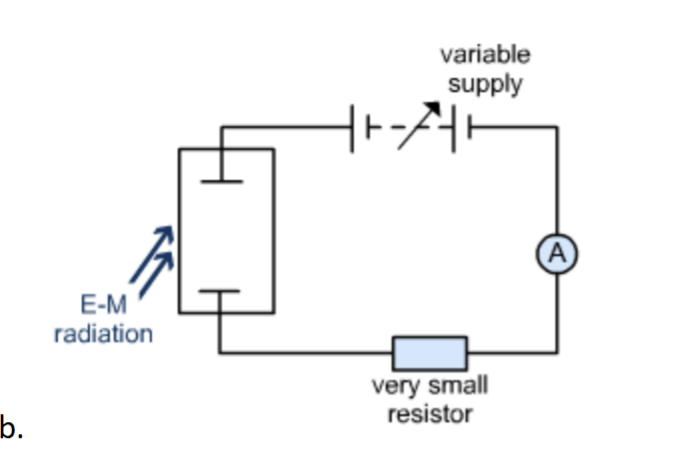
Define stopping potental.
The minimum potential difference at which photoelectrons do not have enough kinetic energy to pass across the gap.
Vs = KEmax/e
Define the de Broglie wavelength.
The wavelength of a particle that has momentum.
λ = h/p where λ is the de Broglie wavelength, h is Planck’s constant, p is the momentum of the particle (p = mv).
Define energy level.
The discrete allowed energy of an electron, within an atom.
Explain why energy levels of electrons in atoms are negative.
A just free electron has zero energy.
In order for the electron to move up energy levels to be released, it must gain energy.
Explain origin of line spectra (spectral lines/emission lines) specific to certain elements at specific frequencies/ wavelengths.
Electrons exist in discrete energy levels.
Elecrton within atom are excited to higher energy level when: fast moving electron collides with atom, transferring its kinetic energy OR current is passed through OR gas is heated.
The electron then falls back down to lower energy level.
Emitting a photon with an energy E, which is equal to the energy difference. ∆E between the two electron levels.
E= hf is the energy of the photon OR E = hc/λ is the energy of the photon.
The photon is emitted with a specific frequency f =∆E / h where ∆E is the energy difference between the levels OR The photon emitted with a specific wavelength λ=hc /∆E where ∆E is the energy difference between the levels.
There are only a limited number of energy differences between levels and there are only a corresponding limited number of frequencies/wavelengths of photon emitted.
Different elements have different energy differences between levels, so produce different spectral lines.
Explain origin of absorption spectra at specific frequencies/wavelengths.
Electrons exist in discrete energy levels.
Electron within atom (e.g. in atmosphere of sun) excited to higher energy level when it absorbs a photon. (DO NOT STATE HOW)
E = hf or E = hc/λ is the energy of a photon.
The photon absorbed must have a specific frequency f =∆E / h where ∆E is the energy difference between the levels OR The photon absorbed must have a specific wavelength λ=hc /∆E where ∆E us the energy difference between the levels.
There are only a limited number of energy differences between levels and only a corresponding limited number of frequencies/wavelengths of photons absorbed.