The citric acid cycle
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
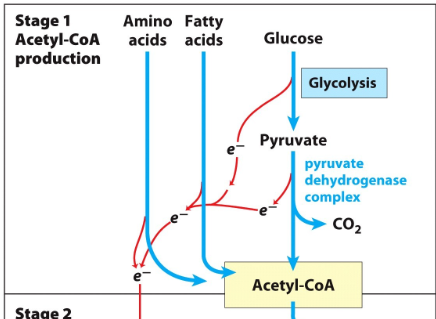
Stage 1 of cellular respiration
Organic fuel molecules such as glucose fatty acids and some amino acids are oxidized to yield 2 carbon fragments in form of acetyl- CoA
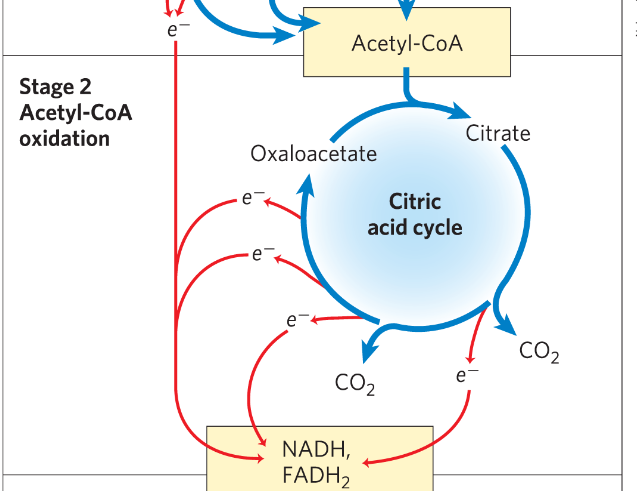
Stage 2 of cellular respiration
The acetyl groups are oxidized to CO2 in the citric acid cycle
Energy of the oxidation is conserved in electron carriers NADH and FADH2 by reduction
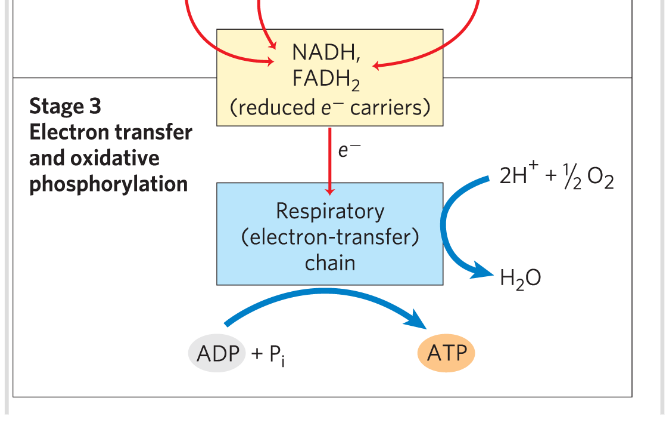
Stage 3 of cellular respiration
The reduced coenzymes are themselves oxidized giving up protons H+ and electrons
Electrons are transferred to O2 via a series of electron carrying molecules - in the respiratory chain- forming water
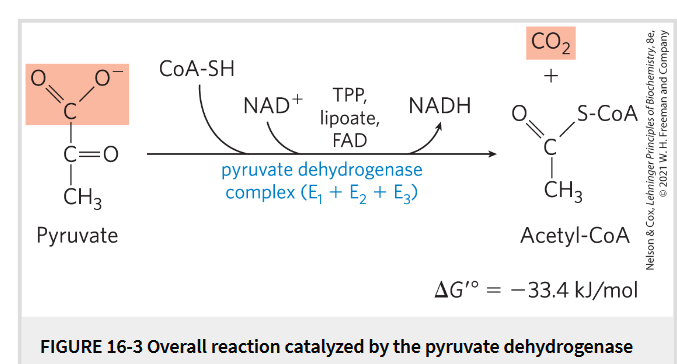
Pyruvate
The metabolite that links 2 central catabolic pathways- glycolysis and citric acid cycle→ a logical point of regulation that determines the rate of catabolic activity
Summarize what the citric acid cycle does
Oxidizes acetyl CoA to CO2 and H2O
Energy from the oxidation drives the synthesis of ATP
Conserving energy
Citric acid cycle is a hub if metabolism
Catabolic pathways in and anabolic out
breakdown products of many amino acids and nucleotides are intermediates in the cycle that can be fed in
How can the citric acid cycle be regulated?
Can be regulated by allosteric and covalent mechanisms to achieve homeostasis
Production of acetyl - CoA
CoA has a reactive thiol (SH) group covalent linked and critical to its role as an acyl carrier (thioester)
High standard free energies of hydrolysis→ high acyl group transfer potential
Pre-Reaction: Pyruvate→ acetyl CoA
Pyrivate is oxidized to acetyl CoA+ CO2
Occur in mitochondria and transported by MPC
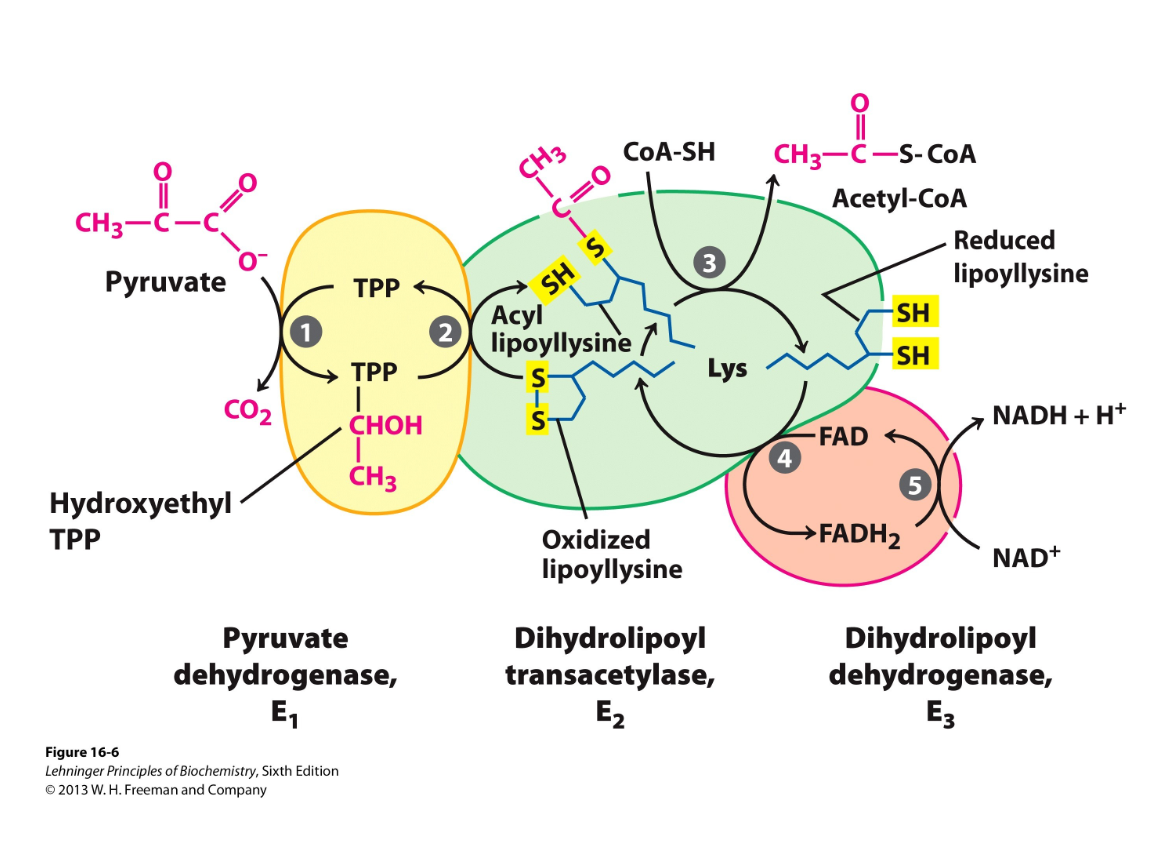
By pyruvate dehydrogenase (PDH) complex- several cofactors involved: 3 enzymes and 5 coenzymes
Reaction:oxidative decarboxylation
Irreversible
NADH formed
PDH complex
3 enzymes- E1,2,3
5 coenzymes: TPP, CoA, FAD, NAD, lipoate
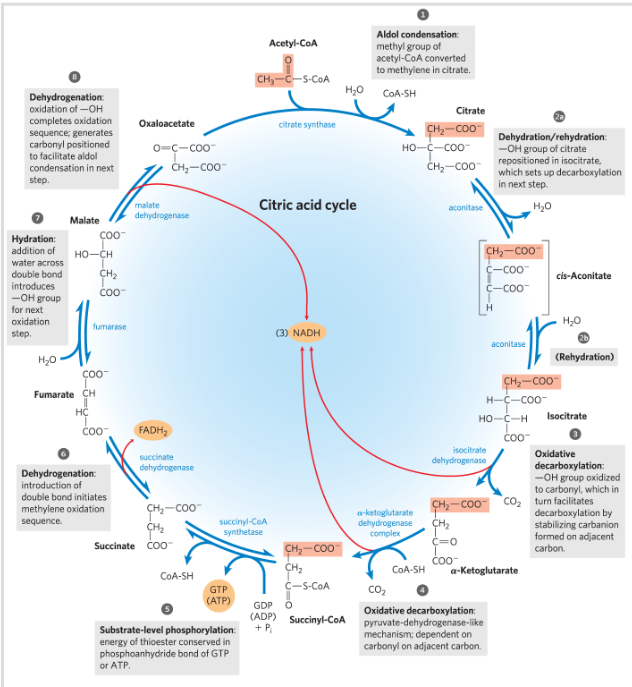
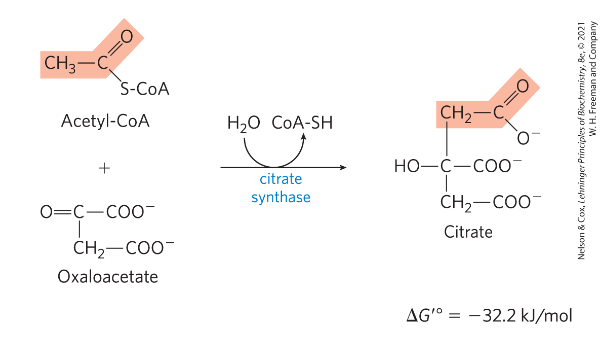
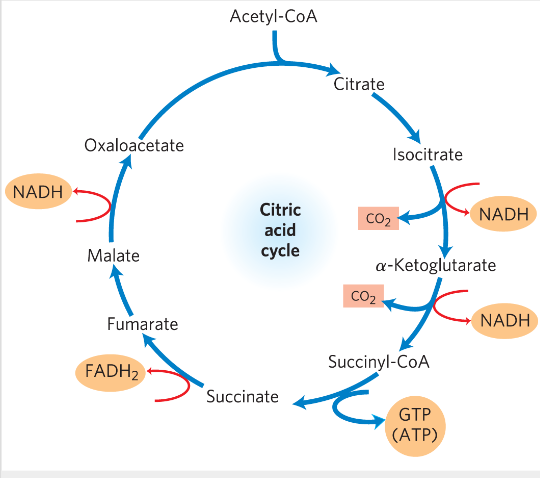
Reaction 1: condensation of acetyl CoA with oxaloacetate
1) OAA + Acetyl-CoA -> citrate + CoA
*) catalyzed by citrate synthase - induced fit-> example (conformational changes occur in substrate and enzyme- catalytic activity) dimer
*) "irreversible" step-> larger negative free energy change
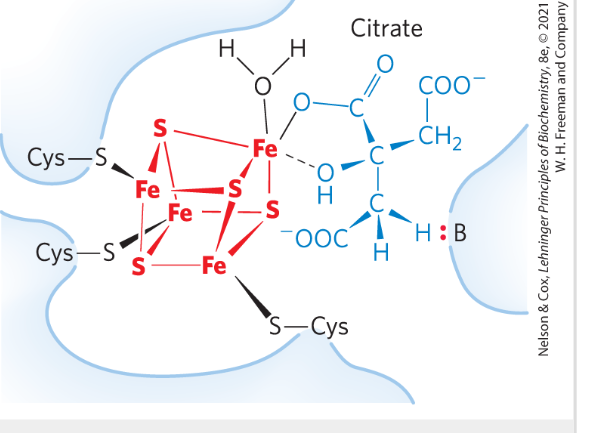
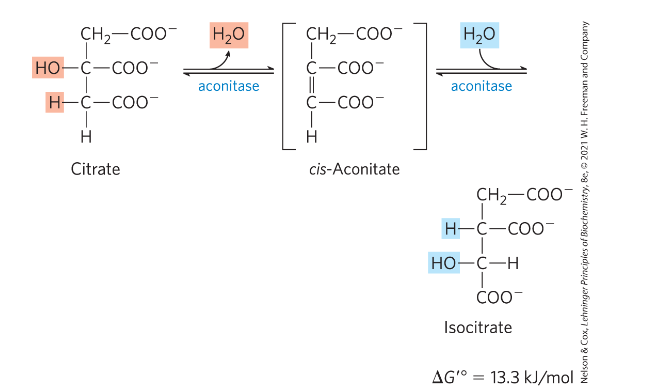
Reaction 2: Formation of Isocitrate via cis-Aconitate
Reaction 2: Isomerization of citrate to iso-citrate
2 step process-> goes via an intermediate cis-Aconitate (not stable)-> isocitrate
Aconitase-> catalyzes hydroxylation from "correct" side of cis-aconitate
- acheived through a cofactor: iron-sulphur FeS complex (very unusual!)-> orients the correct addition of water (binding the substrate in correct orientation)
FeS→interactions points
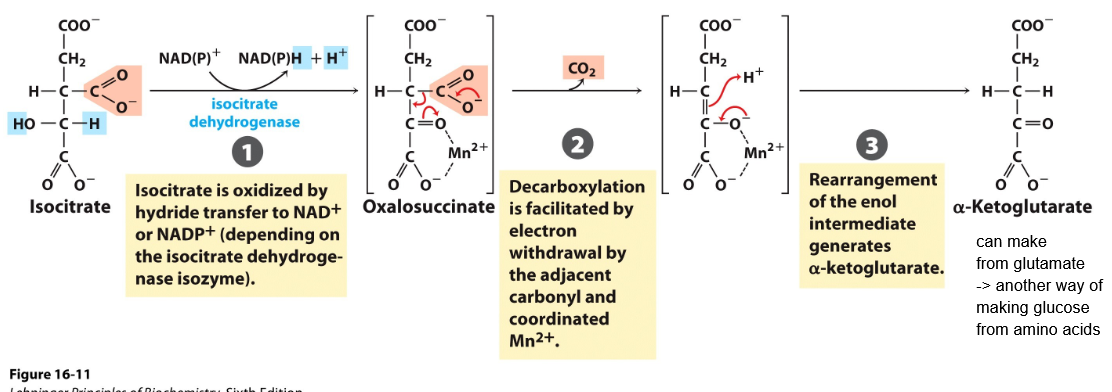
Reaction 3: Oxidation of Isocitrate to α- Ketoglutarate and CO2
Reaction 3. Oxidated decarboxylation
Isocitrate-> alfa-keto glutarate (alfaKG)
Electrons are captured on NADH + H+
CO2 is formed
ENzyme: isocitrate dehydrogenase - "irreversible" step
The middle carboxyl group is expelled
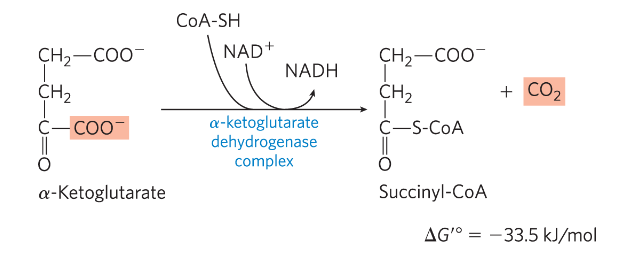
Reaction 4: Oxidation of α-Ketoglutarate to
Succinyl-CoA and CO2
Reaction 4. aKG-> succinyl CoA
Need CoA
*) enzyme aKG DH - similar to pyruvate DH, E3 identical between PDH and aKG DH
*) Irreversible step
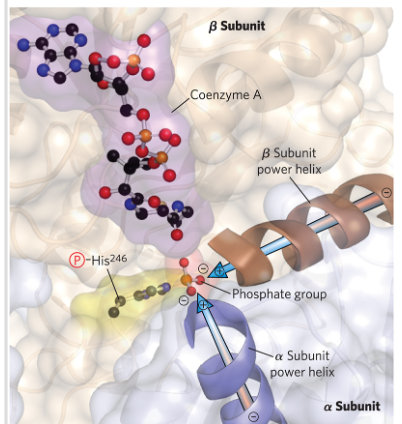
Reaction 5: conversion of succinyl-COA to succinate
Reaction 5: Succinyl-CoA -> Succinate + CoA
*) Succinyl CoA synthetase (tase ending-> GTP involved instead of ATP)
- The “power helices” from the α and β subunits are positioned so their dipoles (+) point toward the negatively charged phosphate, stabilizing it electrostatically.
In this case- stabilizes a phospho-histidine
*) substrate level phosphorylation of GDP to GTP (GTP is equivalent to ATP -><-)
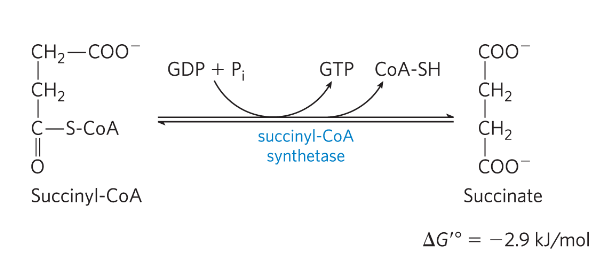
Reaction 6: Oxidation of succinate to fumarate
Reaction 6: Oxidation of succinate->fumarate
Electrons on FADH2
*) enzyme succinate dehydrogenase
- membrane protein (mitochondrial inner membrane)
Electrons from succinate-> electron transport chain
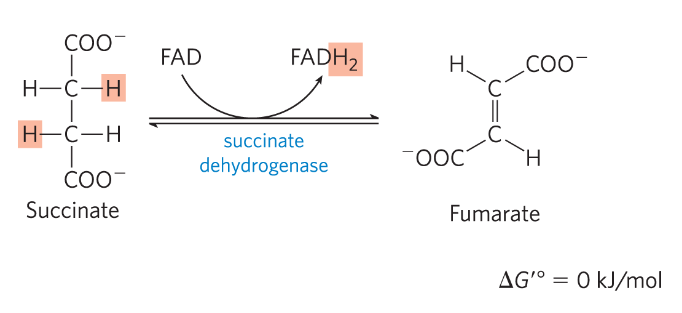
Reaction 7: Hydration of fumarate to malate
Fumarate-> L-Malate
Water is added
*) fumarase
- stereoselective
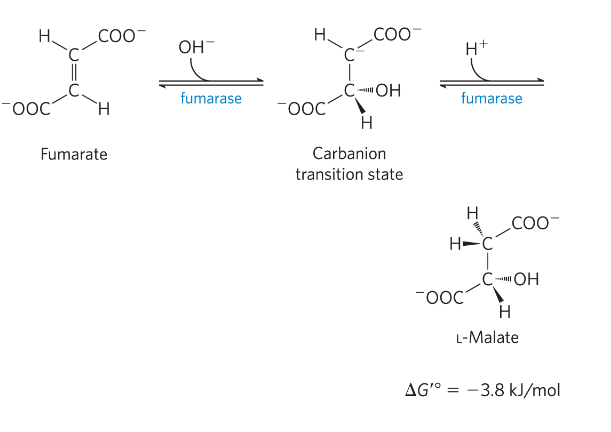
Reaction 8: Oxidation of malate to OAA
Hydride transfer
*) malate dehydrogenase (DH)
Reversible-> unfavorable reaction
Mitochondrial matrix conditions deltaG=close to 0- reversible (low [OAA] in cells)→ pulling the reaction toward OAA
![<p>Hydride transfer</p><p>*) malate dehydrogenase (DH)</p><p>Reversible-> unfavorable reaction</p><p>Mitochondrial matrix conditions deltaG=close to 0- reversible (low [OAA] in cells)→ pulling the reaction toward OAA</p>](https://knowt-user-attachments.s3.amazonaws.com/30a3328c-ca3d-41d9-82e5-42ea696e8018.png)
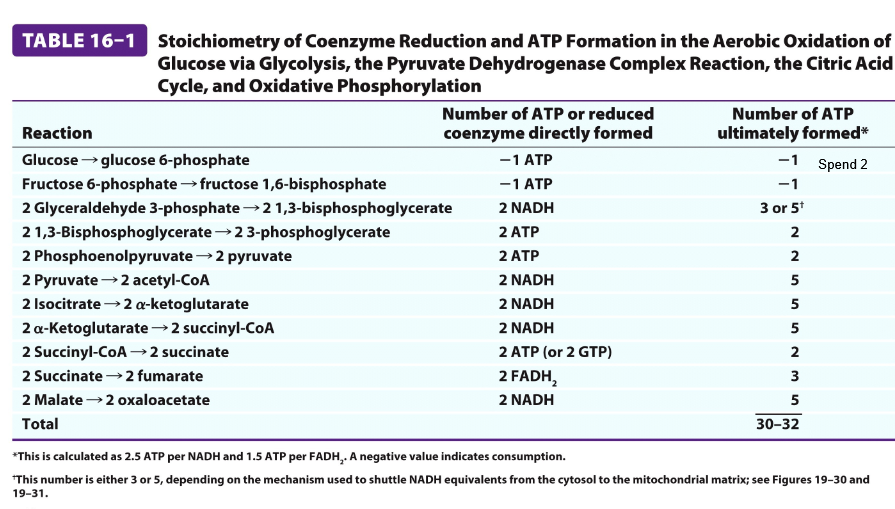
SUmmary CAC
OAA is regenerated at the end of the cycle
3 NADH, 1 FADH2, 1 GTP/ATP, 2 CO2 / PYRUVATE
Even though only 1 ATP is generated the cycle provides a large flow of electrons into the respirator chain via NADH and FADH2→ leads to the formation of 10 x more ATP
2 Pyruvate=1 GLUCOSE
Amphibolic pathway
The citric acid is an amphipholic pathway that serves in both catabolic and anabolic processes
The citric acid cycle is a hub of intermediary metabolism
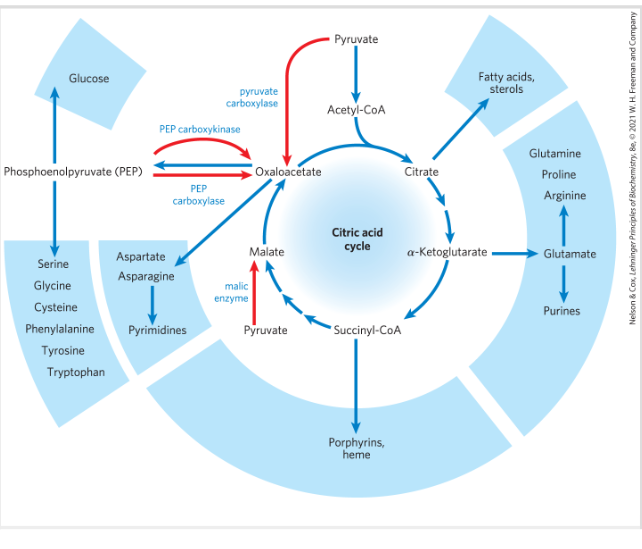
Anaplerotic reactions
REactions that regenerate TCA cycle intermediates-when intermediates like oxaloacetate, malate, or α-ketoglutarate are used for making amino acids, glucose, etc., anaplerotic reactions "refill" them
Most important anaplerotic reaction
Most important: carboxylation of pyruvate by HCO3- → OAA
Catalyzed by pyruvate carboxylase
Requires ATP
Acetyl CoA stimulates the reaction to occur→ if no acetyl CoA no reaction
Requires the vitamin biotin- prosthetic group of the enzyme (activate CO2)- each subunit has a biotin molecule- totally 4
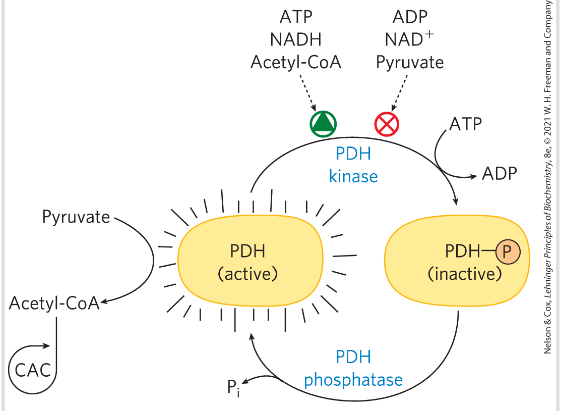
Regulation of citric acid cycle- PDH complex
PDH complex is strongly inhibited allosterically by ATP, acetyl CoA and NADH
PDH complex activity is turned off when ample fuel is available in the fatty acids and acetyl CoA, and when the c ATP is high compared to ADP and NADH higher than NAD+
Alos by P:
PDH-P -> inactive
PDH -> active
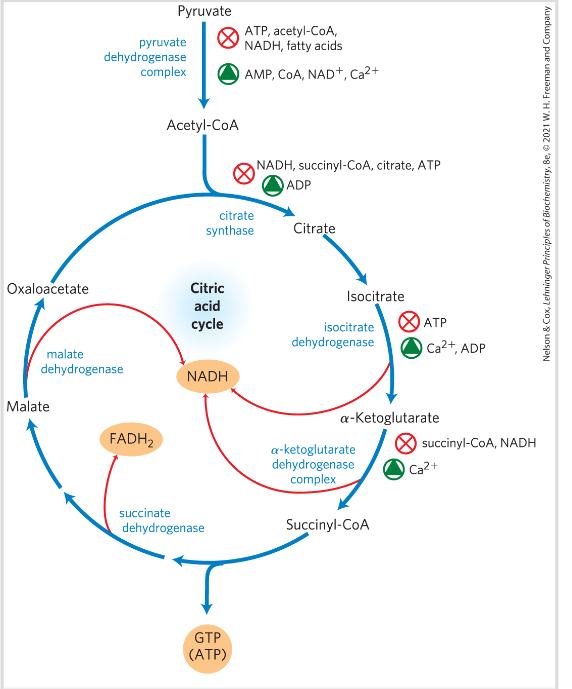
Allosterically regulated enzymes:
- Allosterically regulated enzymes:
*) PDH
*) citric synthase
*) Isocitrate DH
*) aKG DH
Metabolons
a multienzyme complexes
Dilution weakens complexes- separates enzymes
When enzymes in a pathway physically group together, they can pass substrates directly from one to the next — this is called substrate channeling→ faster reactions, more efficient, better control