AP Chem Test 2A
1/15
Earn XP
Description and Tags
Topics 2.1 to 2.4
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
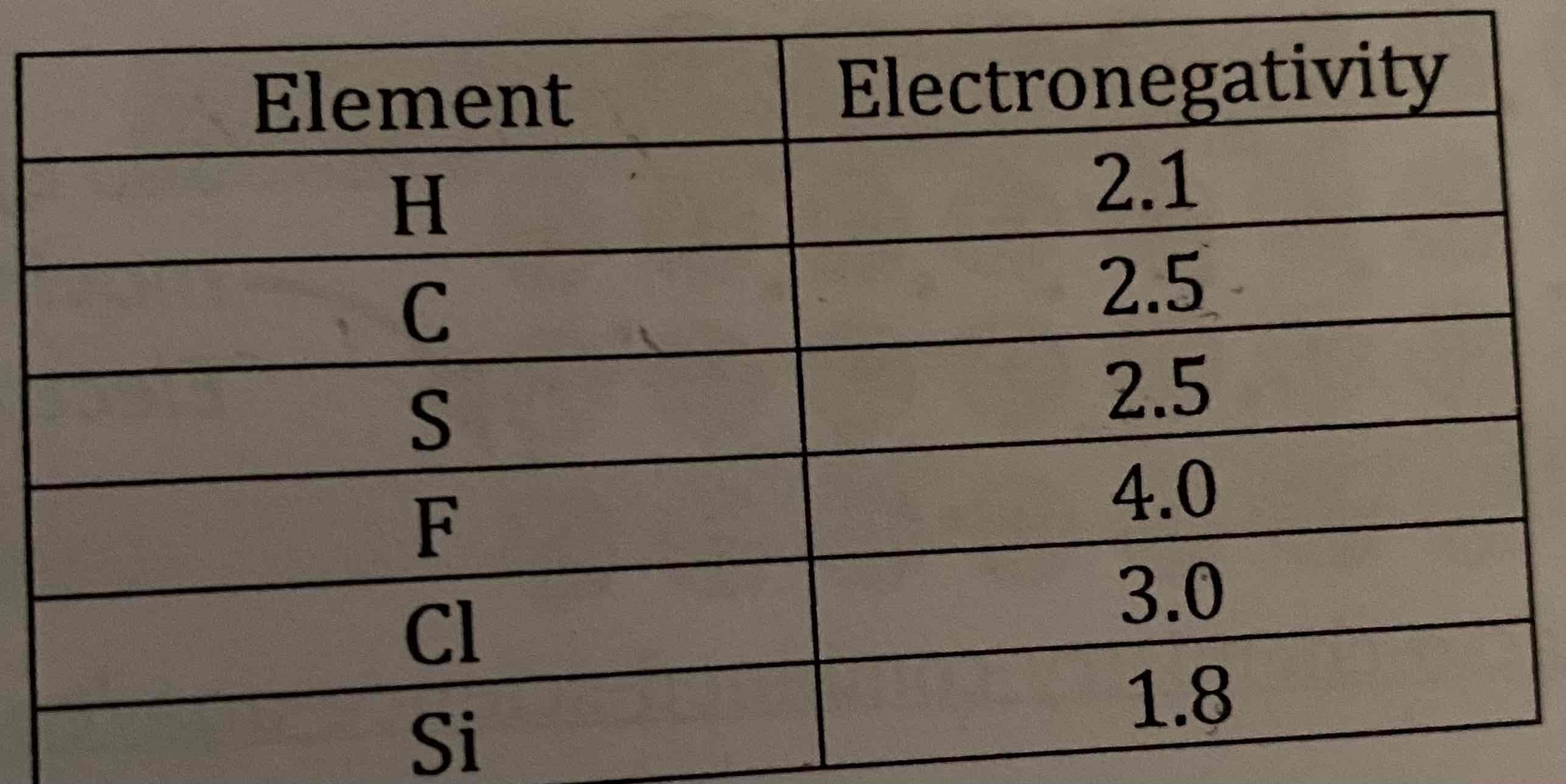
Inspect the table below:
a) Select 3 sets of two elements that would form a polar covalent bond, place them in order from least polar to most polar.
b) Select 3 sets of two elements that would form a nonpolar covalent bond.
a) S-Cl, H-Cl, C-F
b) C-S, H-Si, H-C
Without referencing electronegativity values, place the following in order from least polar to most polar.
a) Si-F, S-F, P-F
b) F-F, F-Br, F-Cl
a) S-F, P-F, Si-F
b) F-F, F-Cl, F-Br
Without referencing electronegativity values, for each of the pairs of elements shown, use a δ- to indicate the area that has a partial negative charge and δ+ to indicate the area of partial positive charge.
a) H-F
b) S=O
c) P-Cl
a) δ+ δ-
b) δ+ δ-
c) δ+ δ-
Based on the locations on the periodic table, rank the following in terms of increasing metallic character.
Ba, Ca, Ge, Se, Ne, Cu, Ga
Ne, Se, Ge, Ga, Cu, Ca, Ba
Choose the substance from each pair that would have the greatest (most exothermic) lattice energy?
A) NaCl vs. LiCl
B) MgO vs. NaCl
C) MgO vs. BaO
D) FeCl2 vs. FeCl3
A) LiCl (smaller size)
B) MgO (greater charge)
C) MgO (smaller size)
D) FeCl3 (greater charge)
Place the following in order from strongest bond energy to weakest.
C-Cl, C-Br, C-F
C-F, C-Cl, C-Br
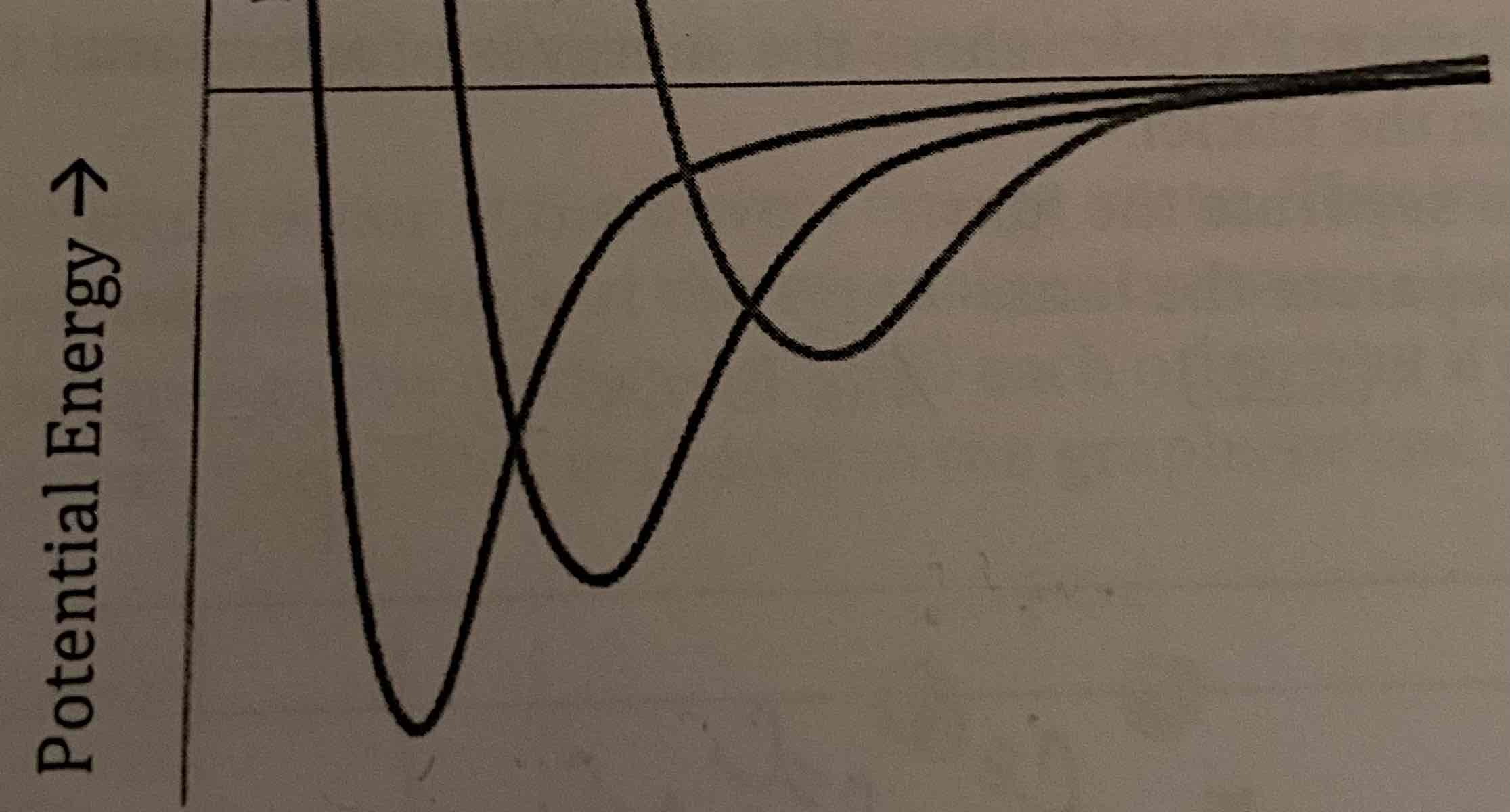
A potential energy diagram for H2, HBr, and Br2 was created but not labeled. Label the lines on the diagram with their corresponding compound.
Biggest Dip: H2
Medium Dip: HBr
Smallest Dip: Br2
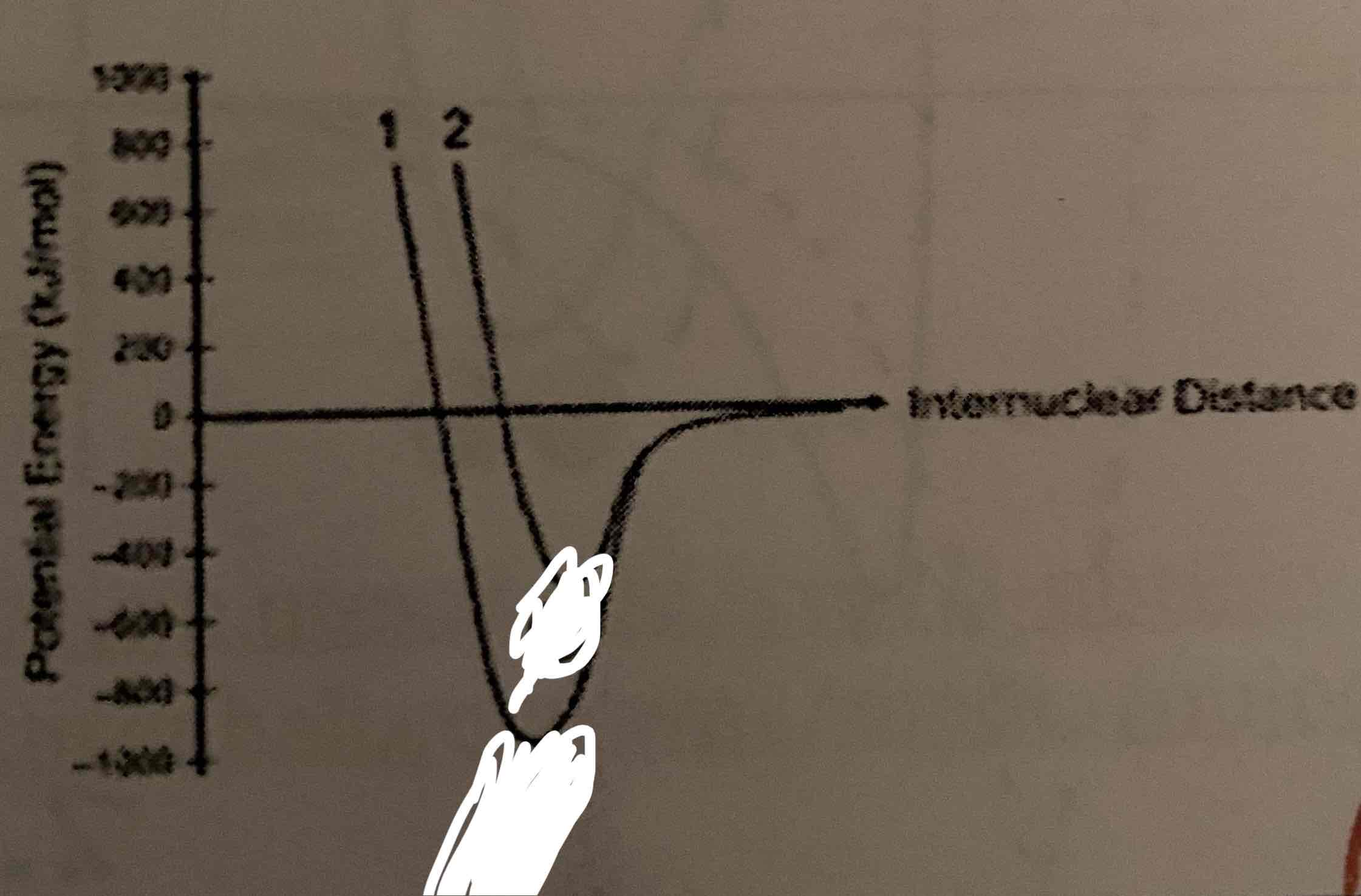
Nitrogen and oxygen form diatomic elements. Nitrogen has a triple bond, while oxygen has a double bond. Identify each on the graph below:
Bigger Dip: Nitrogen (short and strong)
Smaller Dip: Oxygen (long and weak)
An ionic solid does not conduct electricity, but does conduct electricity when dissolved in water. Which of the following explains why?
a. Water is polar so it readily conducts electricity. The ionic solid does not okay a role.
b. The ionic solid has too small of spaces between particles for electricity to flow. Water moves the particles apart enough to conduct electricity.
c. Ionic solids do not have free movement of ions. When dissolved, the ions are free moving. This allows electricity to flow.
d. Ionic solids are too big to conduct electricity. When dissolved, the solid gets smaller and then can conduct electricity.
c. Ionic solids do not have free movement of ions. When dissolved, the ions are free moving. This allows electricity to flow.
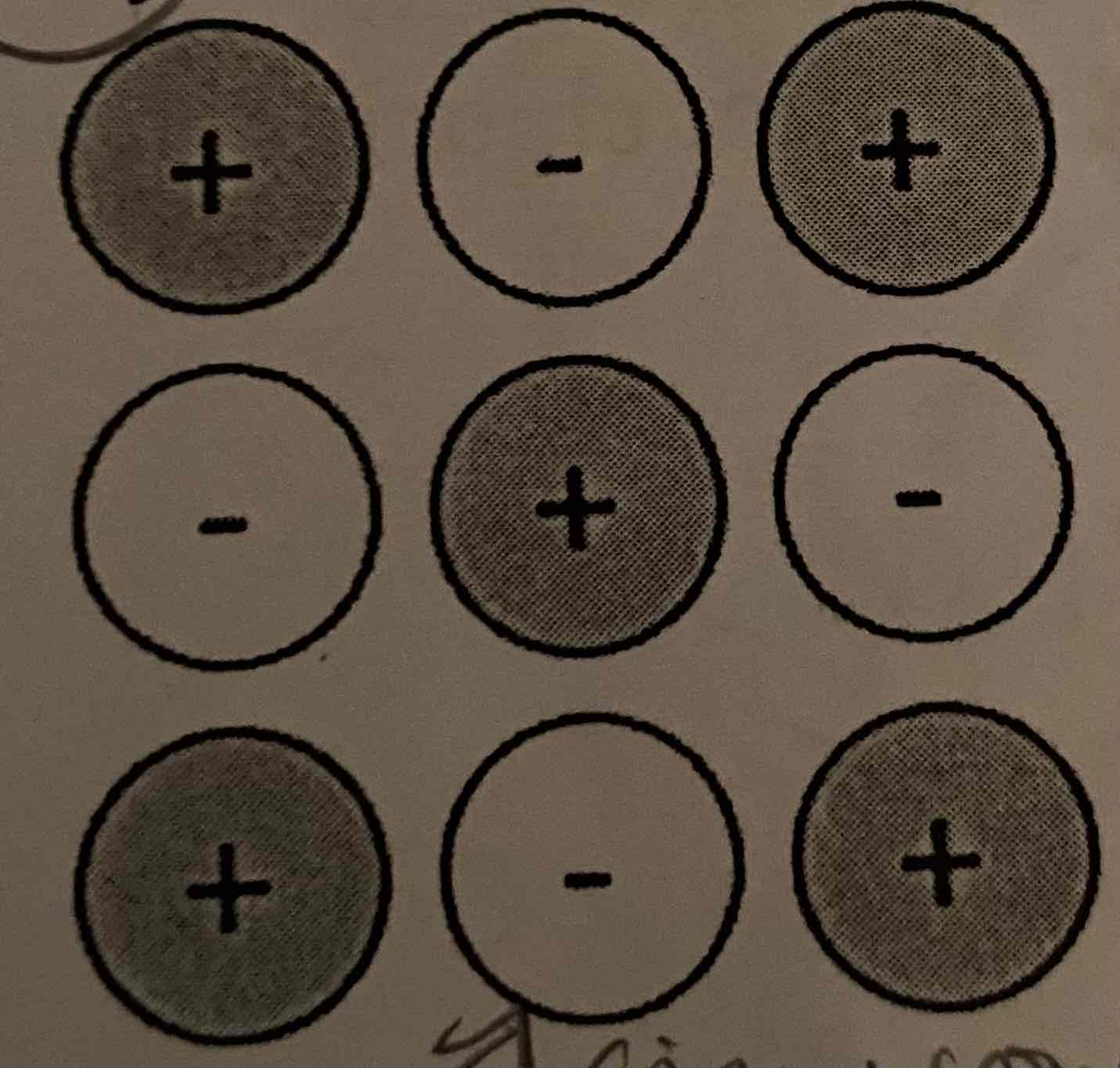
The following particle diagram represents KF. Construct a particle diagram that represents NaCl.
Na (186 pm)
Cl (100 pm)
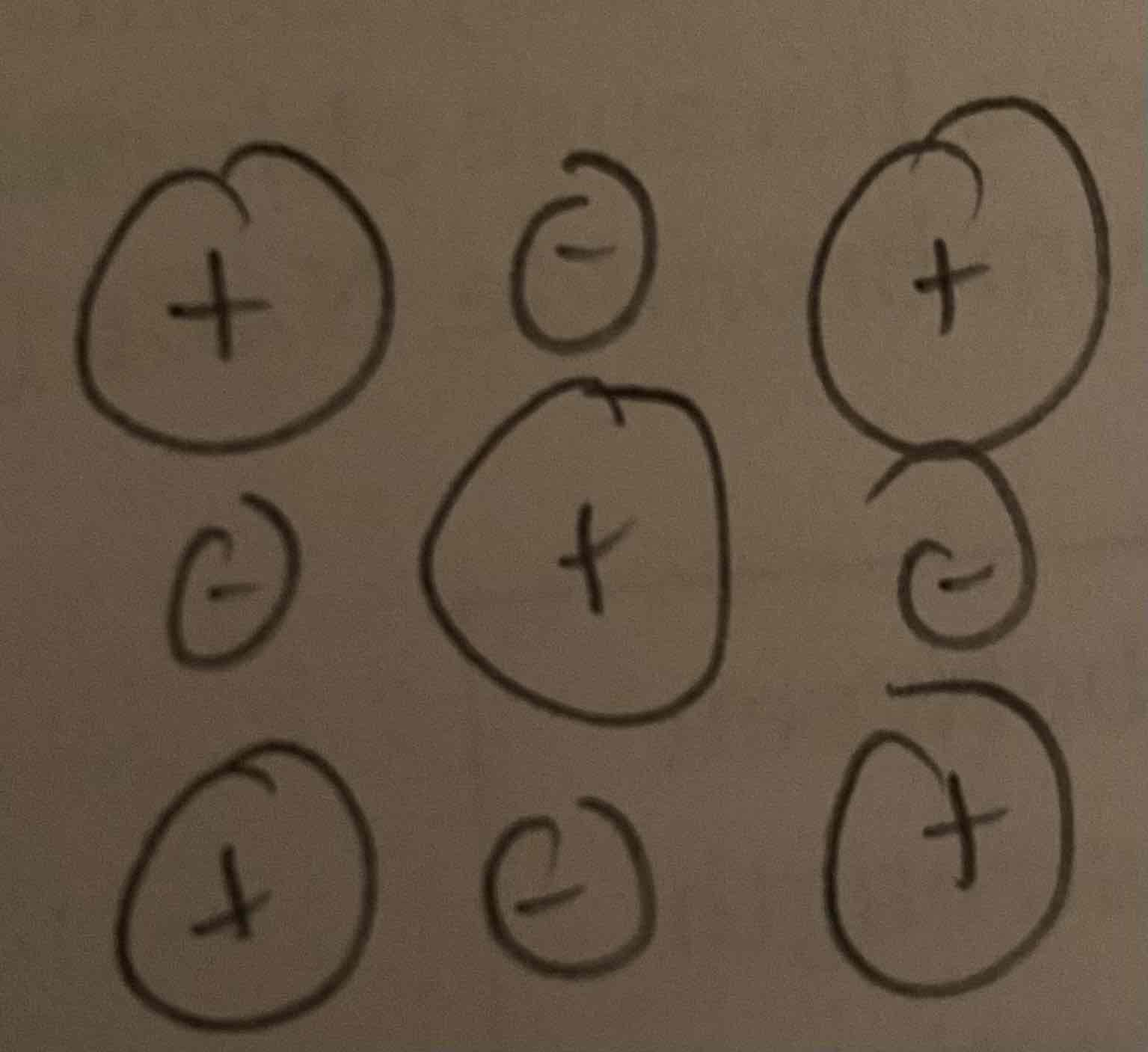
The energy required to dissociate an ionic solid into gaseous ions is known as the lattice energy. Sodium chloride, NaCl, has a lattice energy of 787.3 kJ/mol. Sodium oxide, Na2O, has a lattice energy of 2564 kJ/mol. Which explanation best accounts for this difference?
a. Chloride had a smaller ionic radius than oxide, resulting in a lower lattice energy.
b. Chloride cannot form hydrogen bonds.
c. There are two sodium ions in the chemical formula, meaning there are more ions that need to become a gas.
d. Oxygen has a -2 charge, whereas Cl has a -1 charge. This results in a stronger attraction for Na2O.
d. Oxygen has a -2 charge, whereas Cl has a -1 charge. This results in a stronger attraction for Na2O.
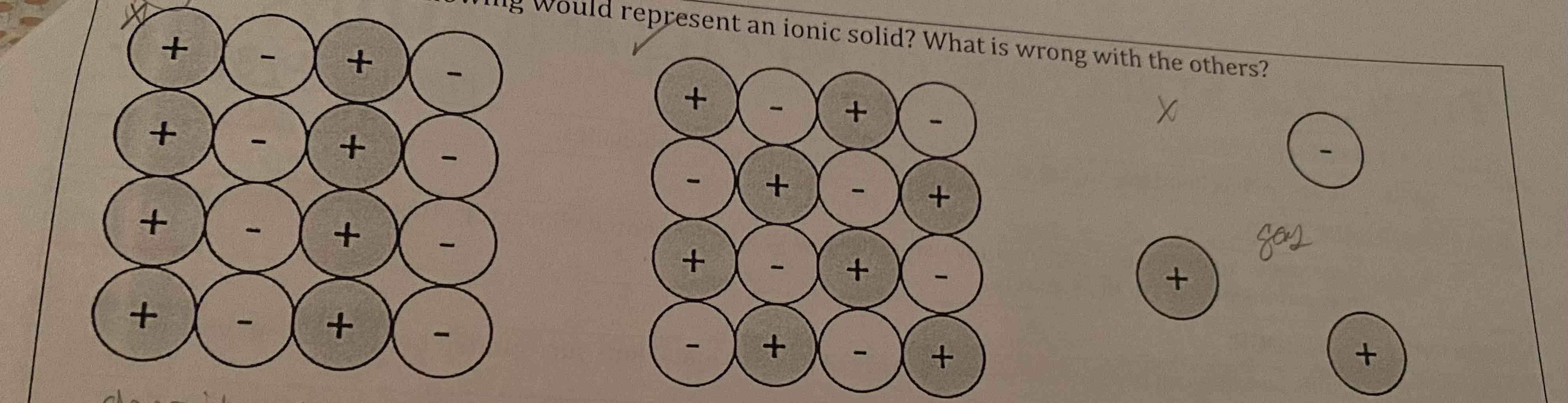
Which of the following would represent an ionic solid? What is wrong with the others?
The first diagram is wrong because oppositely charged ions are not adjacent. The last diagram is wrong because it is not a repeating array, and is therefore a gas. The middle diagram represents an ionic solid.

Below are two examples of alloys, one is an alloy of tungsten with carbon while the other is copper and tin. Label the two types of alloy and create a key to label each type of atom.
(a) Cu and Sn; substitutional; dark atoms are Cu and light atoms are Sn
(b) W and C; interstitial; big atoms are W and small atoms are C
In 2018, scientists engineered an alloy composed of platinum and gold, which is 100 times more wear-resistant than stainless steel. It is 90% platinum and 10% gold. Sketch a model of 20 atoms of this alloy, indicating if it is interstitial or substitutional, use the correct ratio of atoms and be sure to include a key.
It is substitutional because Pt and Au are close in size.
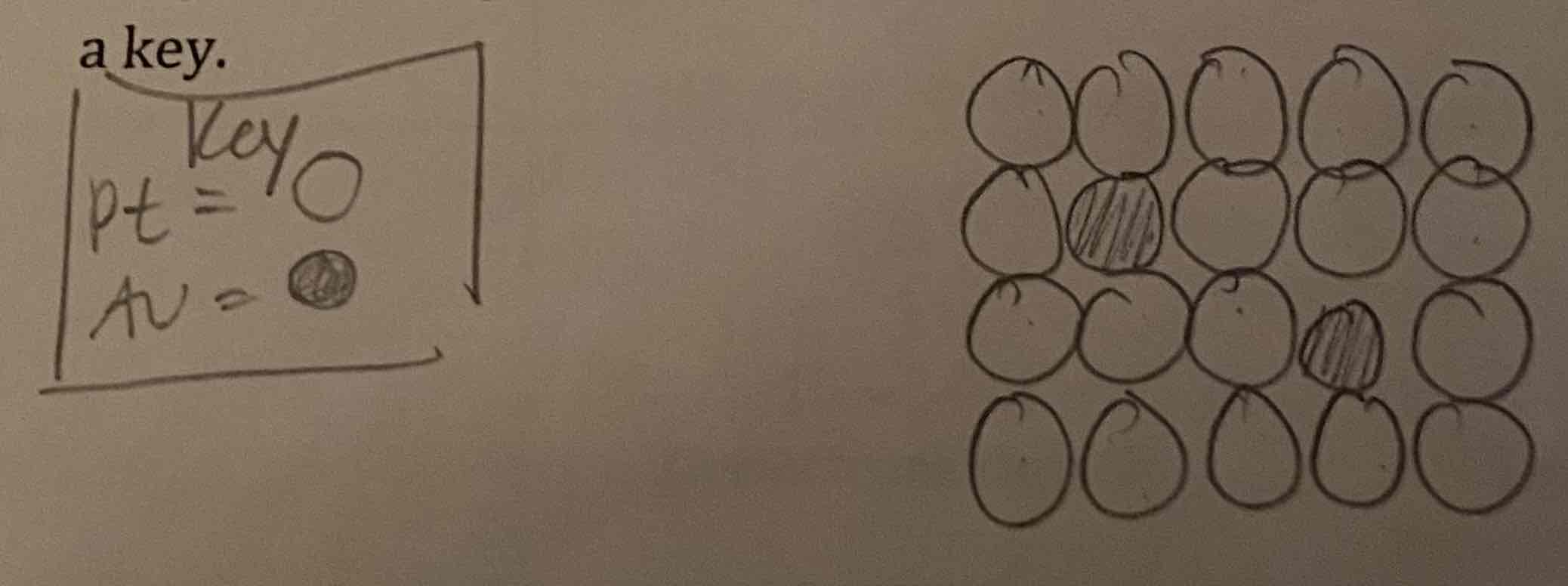
Compare the metals calcium and magnesium, which metal would be stronger (more tightly held together), justify your selection.
Mg would be stronger because it’s smaller than Ca, so its atoms are more tightly packed.
A pure metal and an interstitial alloy containing the same metal were examined. Predict which substance will have a greater density and justify your selection.
I think the interstitial alloy will have a greater density because it’s more tightly packed as smaller atoms can fill open spaces between bigger atoms.