Apologia chem module 4
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
Valence electrons
The elements that exist farthest from an atom’s nucleus. They are the electrons with the highest energy level number.
What is an atom’s chemistry or chemical behavior determined by?
an atom’s, chemistry or chemical behavior is determined mostly by the number of valence electrons it has
What do atoms in the same periodic table column have in common?
Atoms in the same column of the periodic table have the same number of valence electrons and have very similar chemistry or chemical behavior
Octet rule
Most atoms strive to attain 8 valence electrons.
How to tell the number of valence electrons on the periodic table?
For atoms in groups IA-8A, the number of the column in which the atom is located on the periodic table equals the number of valence electrons the atom has.
All of the noble gases have 8 valence electrons except?
helium
Any atom in 1A-8A has the same number of…
valence electrons as the column number.
Who developed Lewis structures?
Gilbert N. Lewis
What do nonmetals try to do in ionic compounds?
In ionic compounds, nonmetals try to gain electrons so their Lewis structures reach the ideal electron configuration with 8 dots around it
What do metals try to do in ionic compounds?
In ionic compounds, metals try to lose electrons so their Lewis structures reach the ideal electron configuration with no dots around them.
What is an Ion?
an atom that has gained or lost electrons, becoming electrically charged.
Cation
A positively charged ion
Anion
A negatively charged ion
Positive cations have the same name as the atom from which they came.
Negative anions add an -ide suffix to the name of the atom from which they came.
How to name Ions
lonization
The process by which an atom turns into an ion by gaining or losing electrons.
Ionization energy
The amount of energy needed to take an electron
away from an atom.
Where is high and low ionization energy on the periodic table?
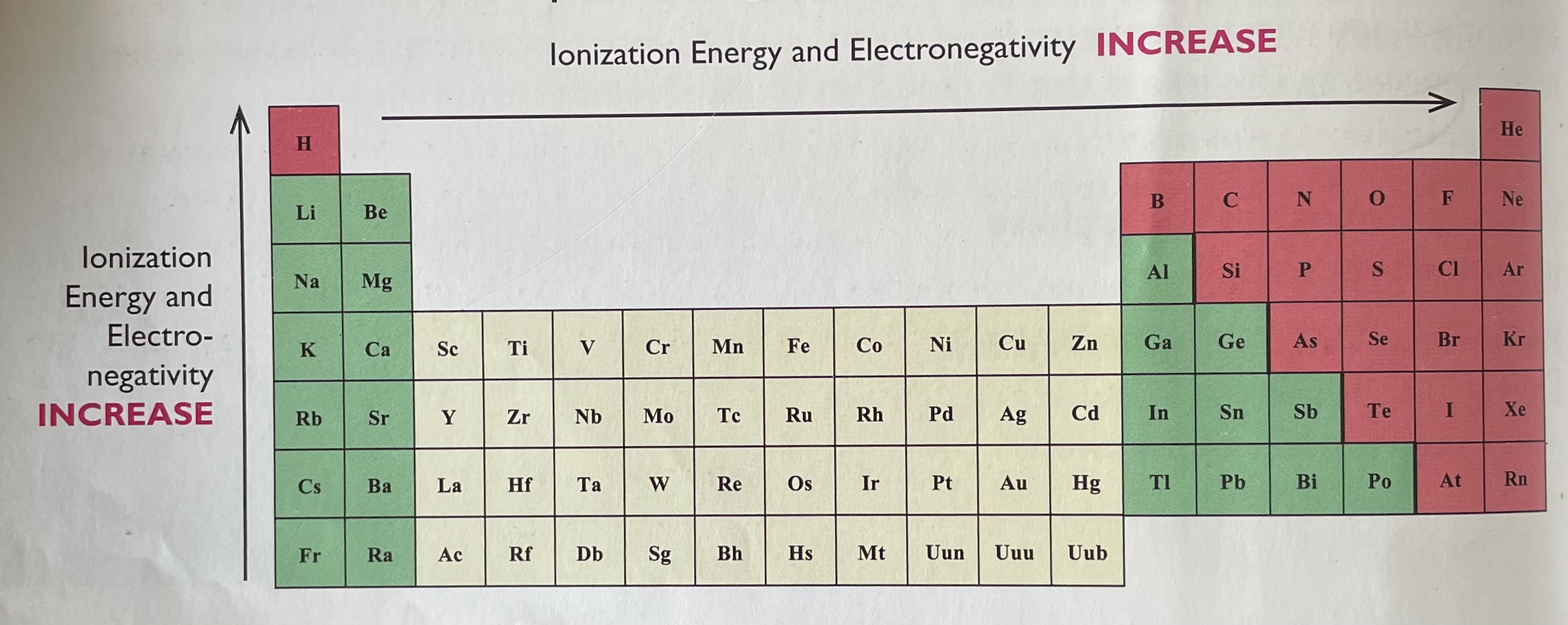
In general, the ionization energy of atoms increases from the left to right and bottom to top on the periodic table
What is a Periodic property?
a characteristic of atoms
that varies regularly
across the periodic table.
What is Electronegativity?
A measure of how strongly an atom attracts extra electrons to itself
where to find electronegativity on the periodic table?
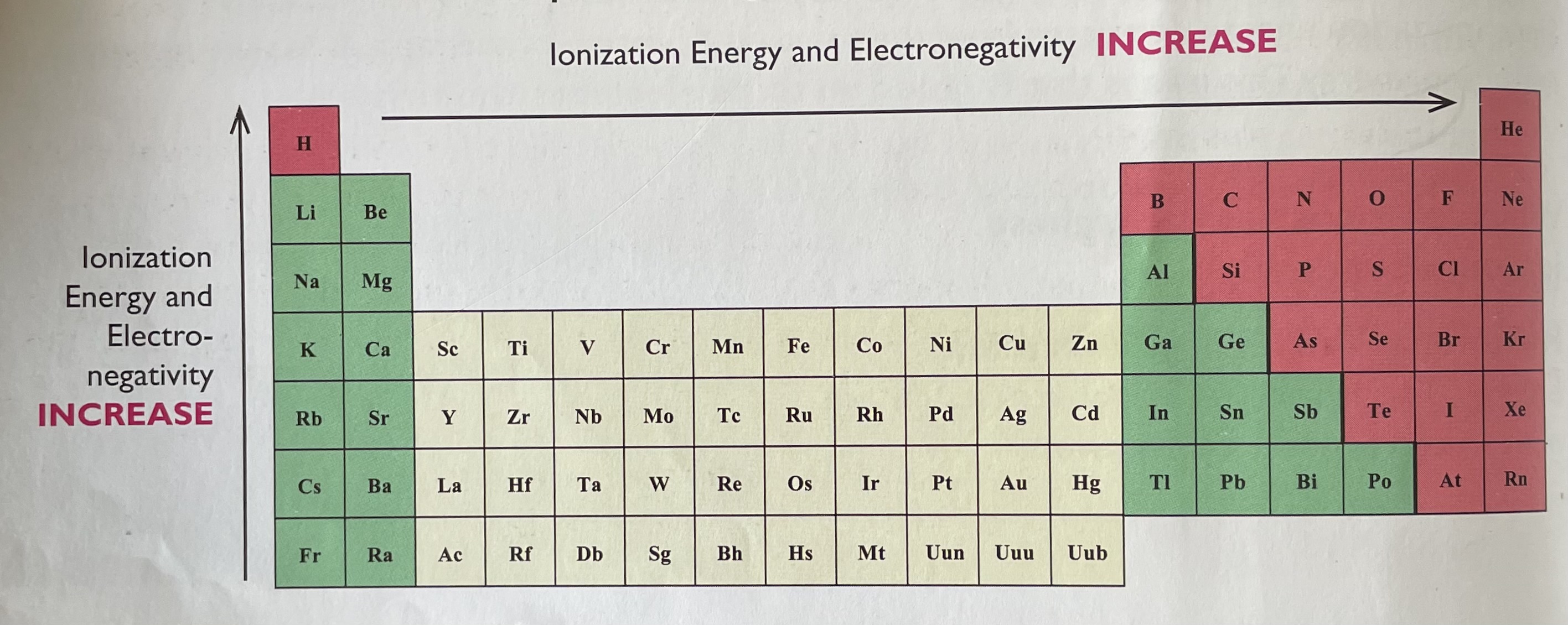
in general electronegativity, of atoms increases as you go from left to right and bottom to top on the periodic table
Where to find atomic radius?
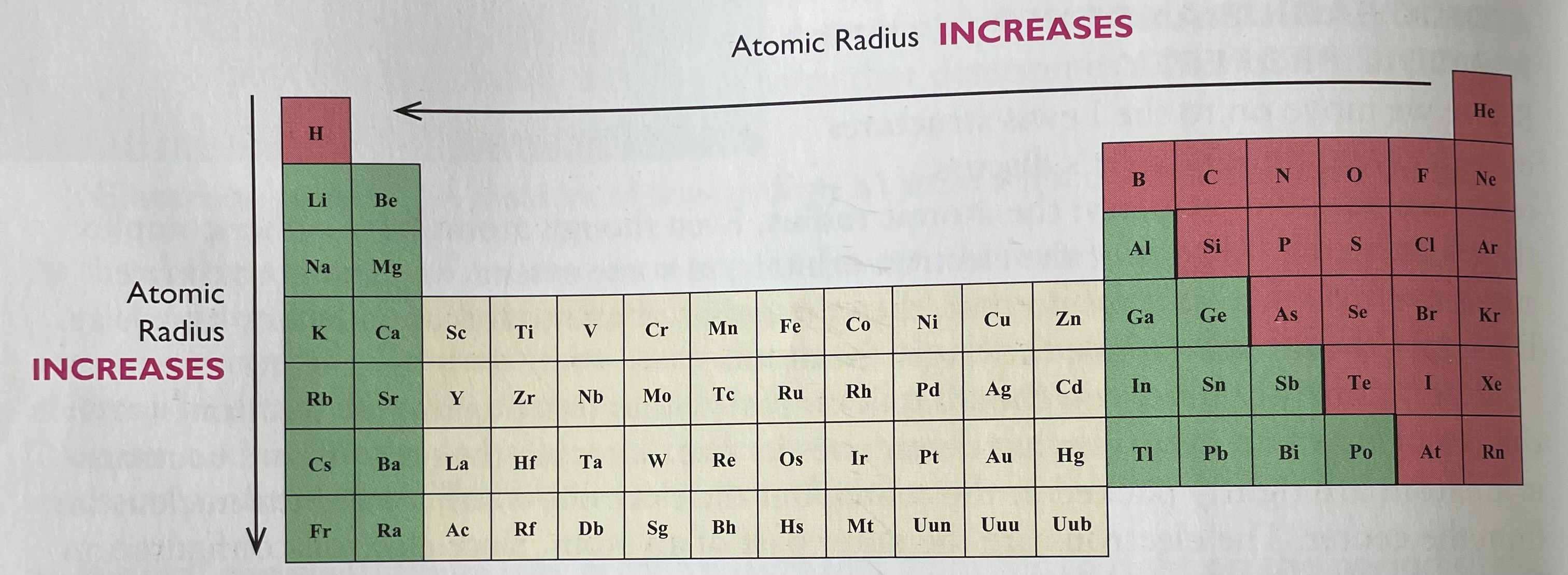
In general atomic radius increases from top to bottom and right two left on the periodic table
What are elements in nature that are NOT composed of individual atoms?
homonuclear diatomics (composed of individual molecules not atoms)
How to find elements made of homonuclear diatomic molecules on the periodic table
look for an upside down “L” from N2itrogen to F2luorine down to Ast2atine then plus H2ydrogen
Covalent bond
A shared pair of valence electrons that holds atoms together in covalent compounds.
Deference between ionic and covalent compounds
The atoms in covalent compounds share electrons to form molecules, while the atoms in ionic compounds give and take electrons to form ions
From bottom to top where are valence electrons closer to the nucleus?
Atoms higher on the periodic table tend to have their valence electrons closer to the nucleus.
The farther an electron is from the nucleus the…
the less attraction the protons can exert on them