Essentials of Biology: Chapter 2
0.0(0)
0.0(0)
Card Sorting
1/36
There's no tags or description
Looks like no tags are added yet.
Last updated 3:00 AM on 9/13/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
37 Terms
1
New cards
Hydrogen bond
Bond that exists between water molecules
2
New cards
Atom
Basic unit of matter
3
New cards
Atomic Number
Number of protons in an atom
4
New cards
Atomic Mass
Number of protons and neutrons in an atom
5
New cards
Isotopes
atoms of the same element, but have a different amount of neutrons
6
New cards
Cohesion
Attraction between particles of the same substance (water→ hydrogen bond)
7
New cards
Adhesion
Attraction between two different substances
8
New cards
Substrate
The substance on which an enzyme acts
9
New cards
Polar
Unequal sharing of electrons
10
New cards
Valence Electrons
Electrons in the outermost orbital of an atom
11
New cards
Covalent bond
Created due to sharing of electrons
12
New cards
Ionic bond
Created due to transferring of electrons
13
New cards
Nucleus
contains Protons and Neutrons
14
New cards
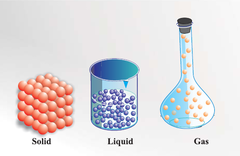
Matter
anything that occupies space and has mass
15
New cards
Element
a substance that cannot be broken down into other substances
16
New cards
Molecule
group of 2 or less atoms bonded together

17
New cards
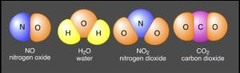
Compound
Molecule containing 2 or more elements in a fixed ration
18
New cards
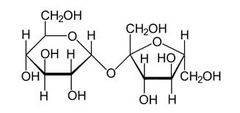
Mixture
2 or more elements mixed in varying rations
19
New cards
Solution
a liquid homogenous mixture of 2 or more elements

20
New cards
Solvent
the dissolving agent

21
New cards
Solute
the substance that is dissolved

22
New cards
Atomic Number
number of protons, same for all atoms of particular element, unique for each element

23
New cards
Mass Number
sum of protons and neutrons

24
New cards
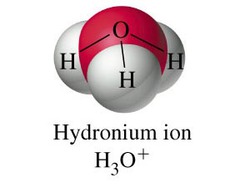
Ion
an atom that has gained or lost an electron
25
New cards
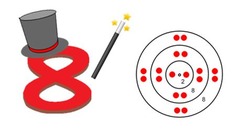
Octet rule
atoms are reactive until they have 8 electrons in each shell
26
New cards
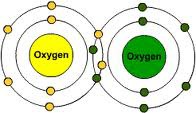
Covalent Bonds
Electron pairs are shared
27
New cards
Nonpolar
both atoms exert equal pull on the shared electrons
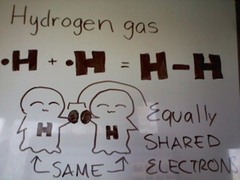
28
New cards
Reactants
Chemicals being changed by a reaction
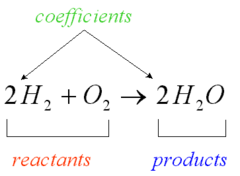
29
New cards
Products
Chemicals produced by a reaction
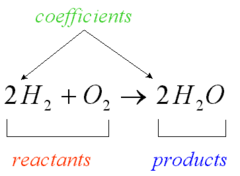
30
New cards
Buffer
Substance that resists changes in pH by accepting or donating H+ ions
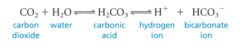
31
New cards
pH Scale
Measurements of the H+ ion/OH- concentrations
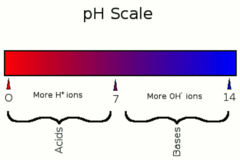
32
New cards
3 subatomic particles and their charges
protons (+), electrons (-), neutrons (0)
33
New cards
Universal solvent
Property of water that allows it to dissolve nutrients and carry them through the body
34
New cards
Electron Shell
Models that show the energy level of electrons
35
New cards
electronegativity
The affinity for electrons in a covalent bond
36
New cards
Hydrophobic
"Water fearing substances" like oil. Repels water usually as a result of non polarity
37
New cards
Hydrophillic
"Water loving substances" like salt. Attracted to water usually due to polar properties.