Alkyne Reagents
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
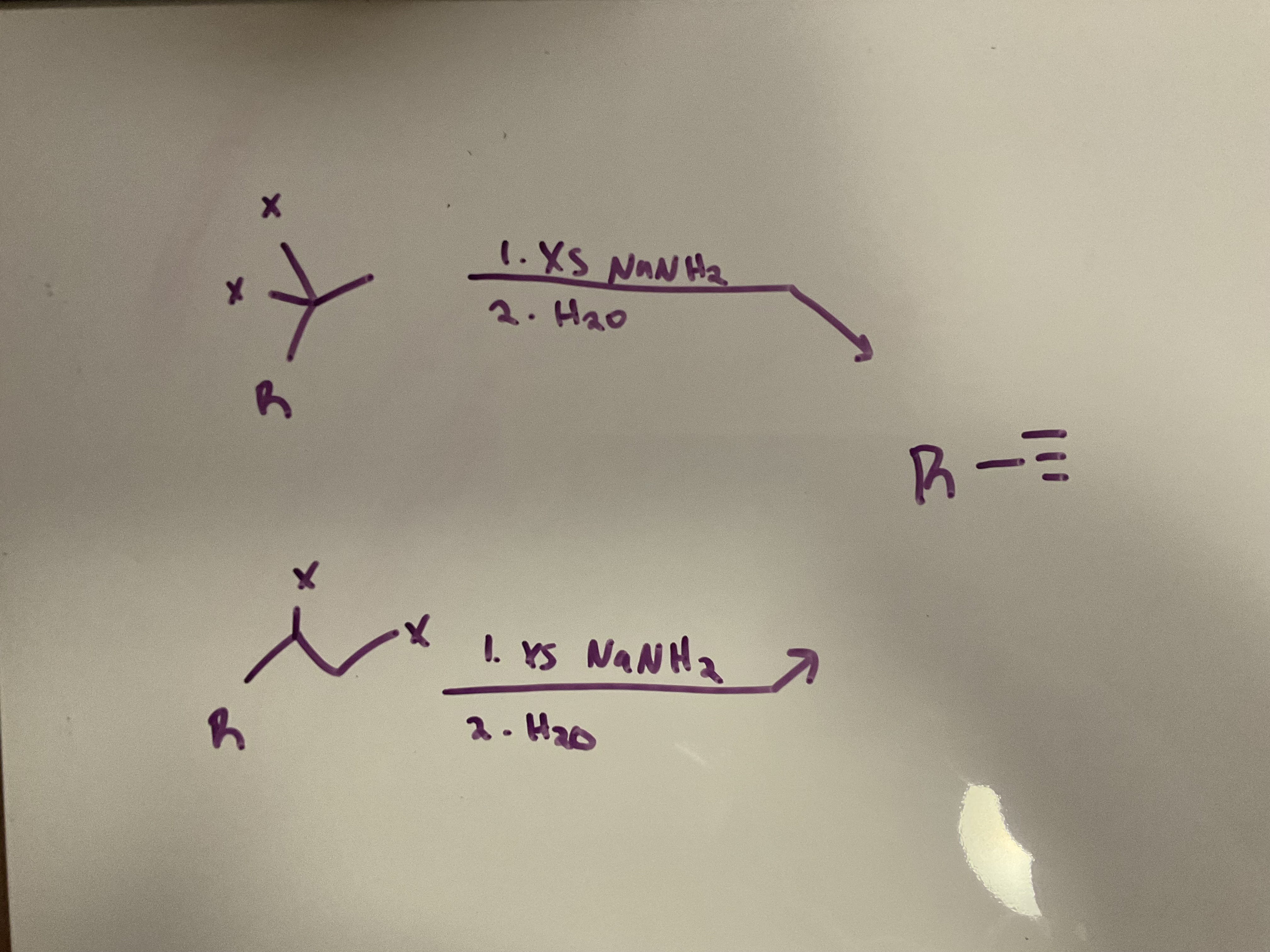
Elimination
Reagents: 1) Excess NaNH2 2) H2O
A vicinal or geminal dibromide is converted to an alkyne
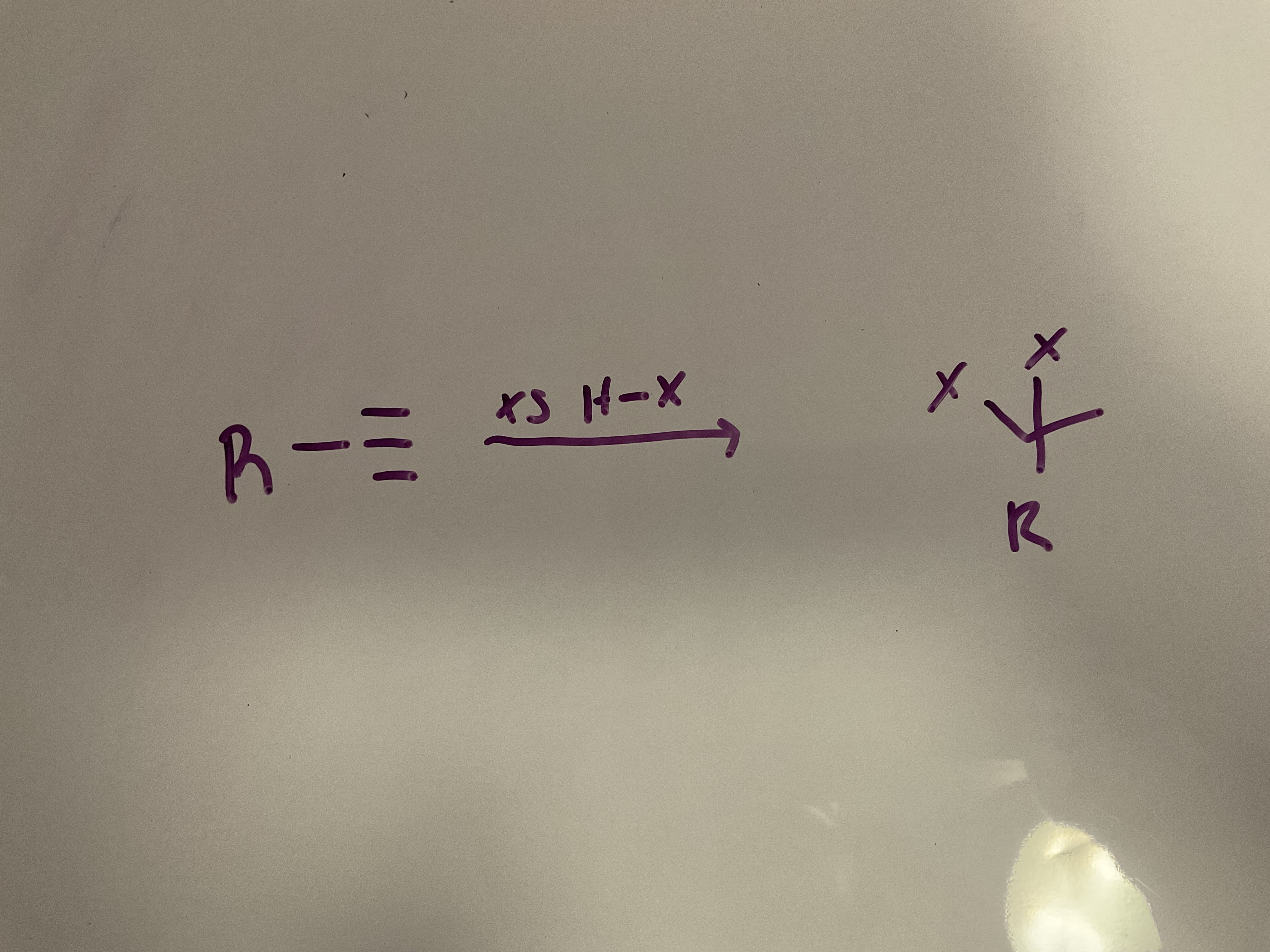
Hydrohalogenation (2 equivalents) : an alkyne undergoes Markovnikov (xs HX gives two addition reactions to give geminal dihalide)
Reagent: HX
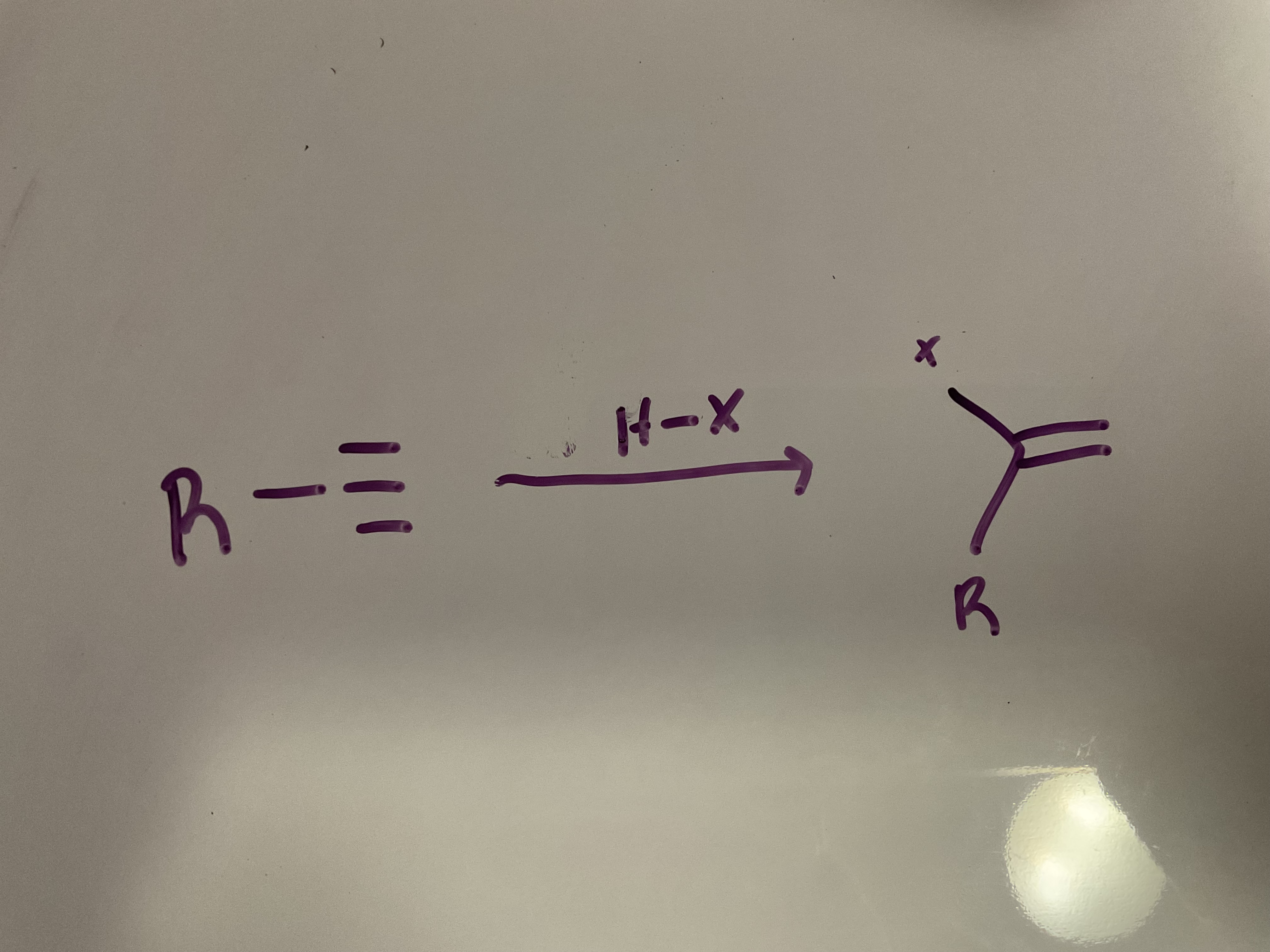
Hydrohalogenation (one equivalent)
Reagent: HX
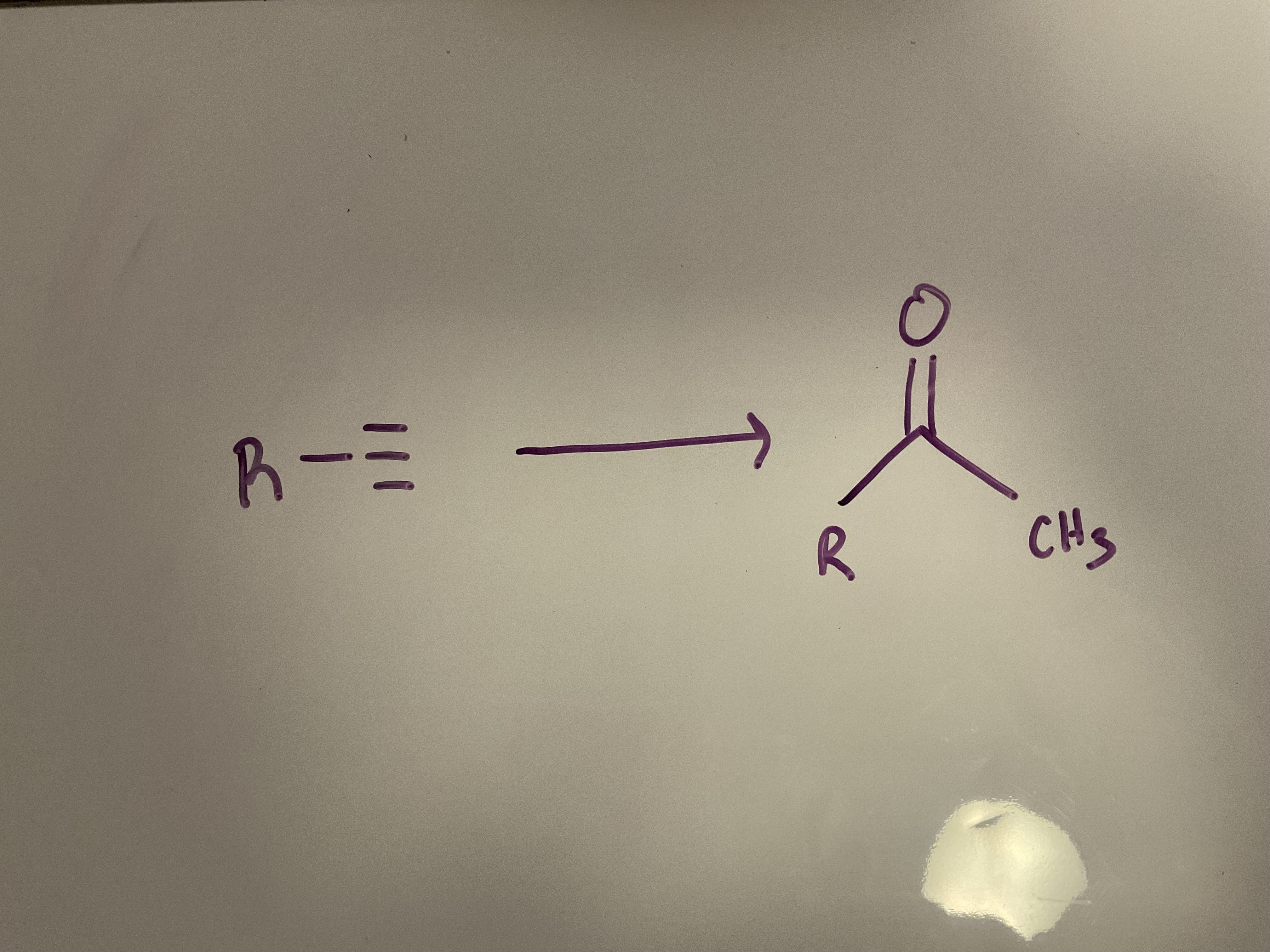
Acid-catalyzed hydration: a terminal Alkynes undergoes Markovnikov addition of H and OH to give an Enol, which quickly tautomerizes to give a ketone
Reagents: H2SO4, H2O, HgSO4
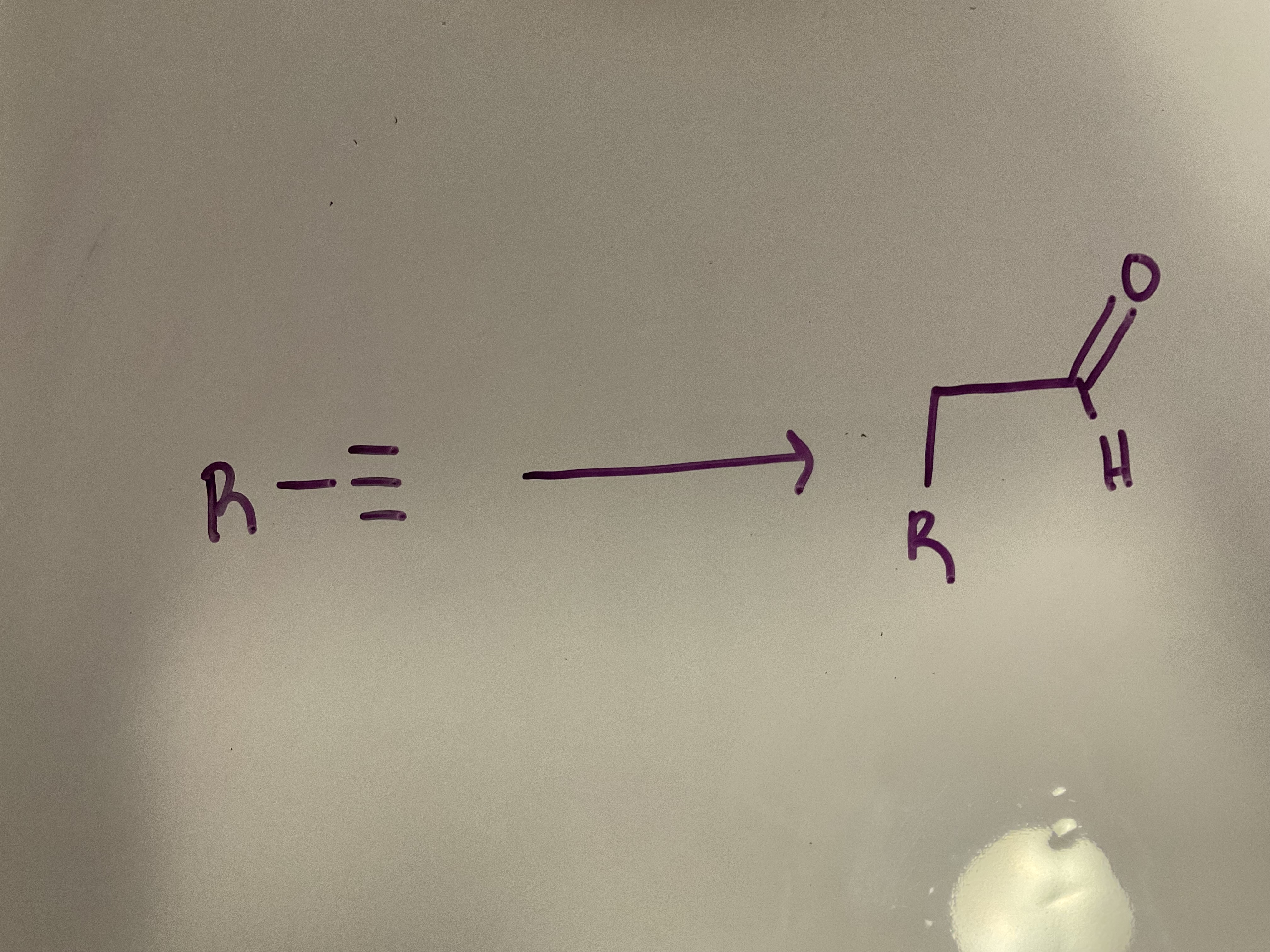
Hydroboration-oxidation: A terminal alkyne undergoes anti-Markovnikov addition of Hand OH to get an enol, then tautomerizes to give an aldehyde
Reagents: 1. R2BH 2. H2O2, NaOH
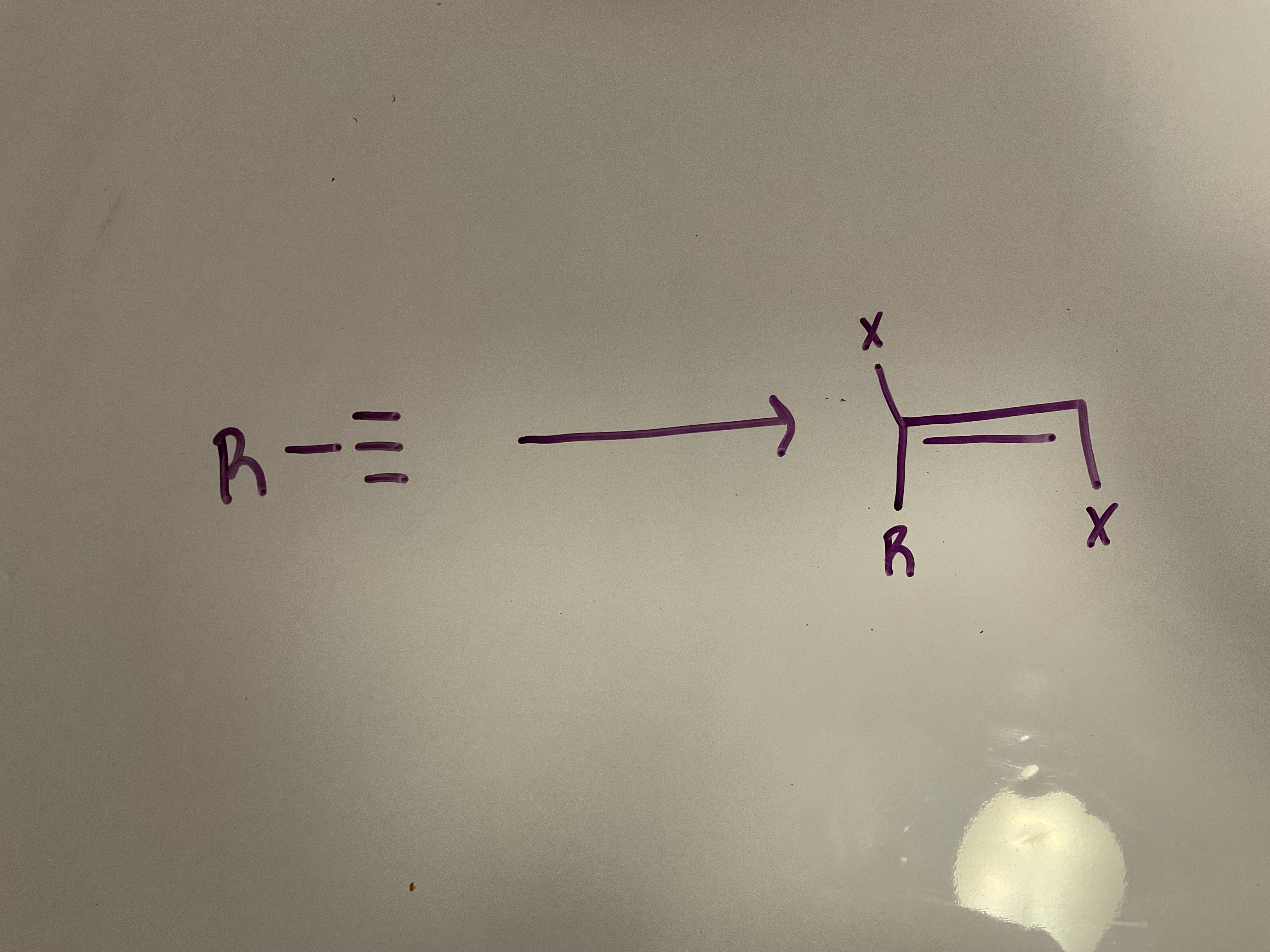
Halogenation
Reagent: X2, CCl4
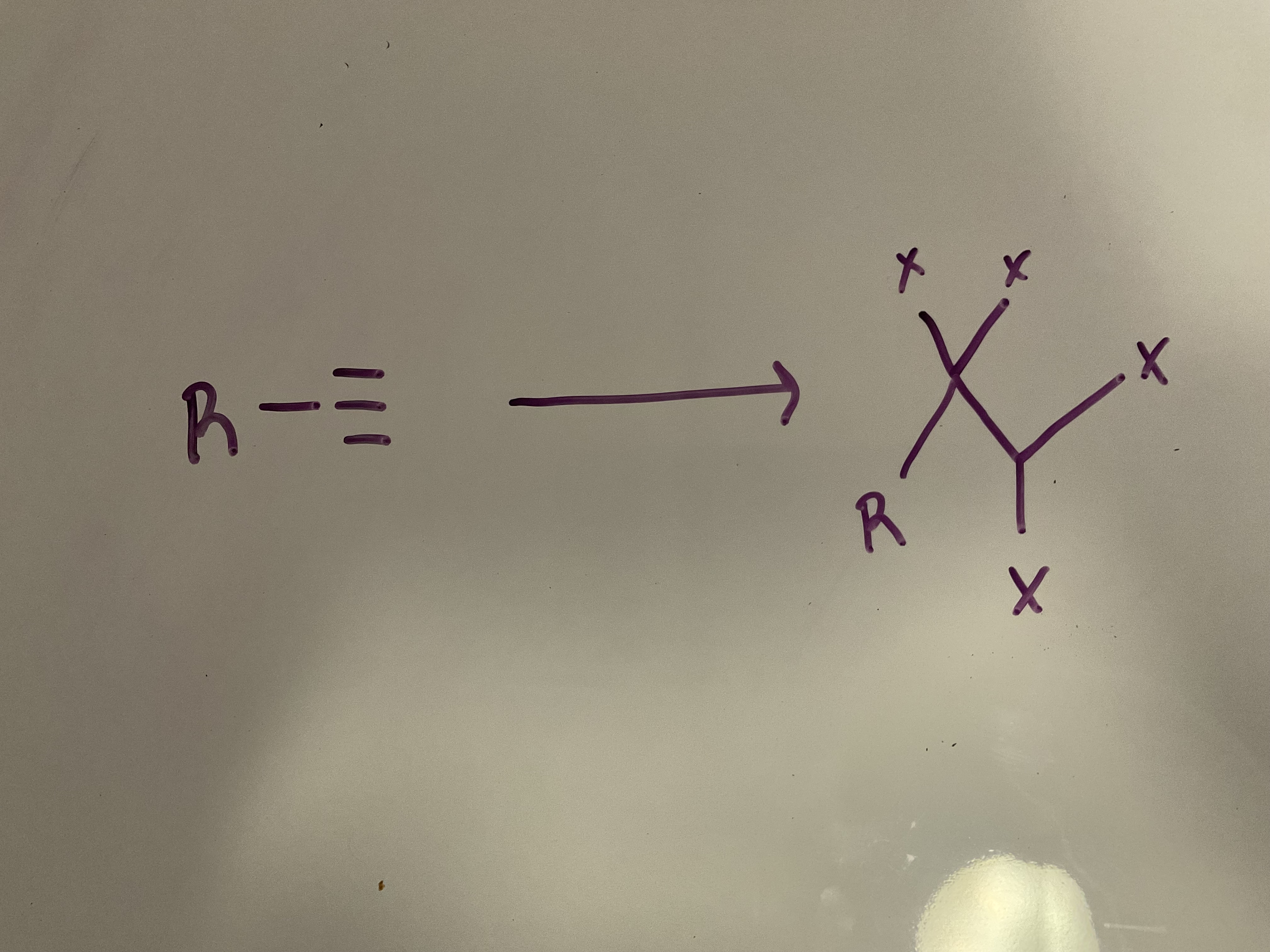
Halogenation
Reagent: xs X2, CCl4
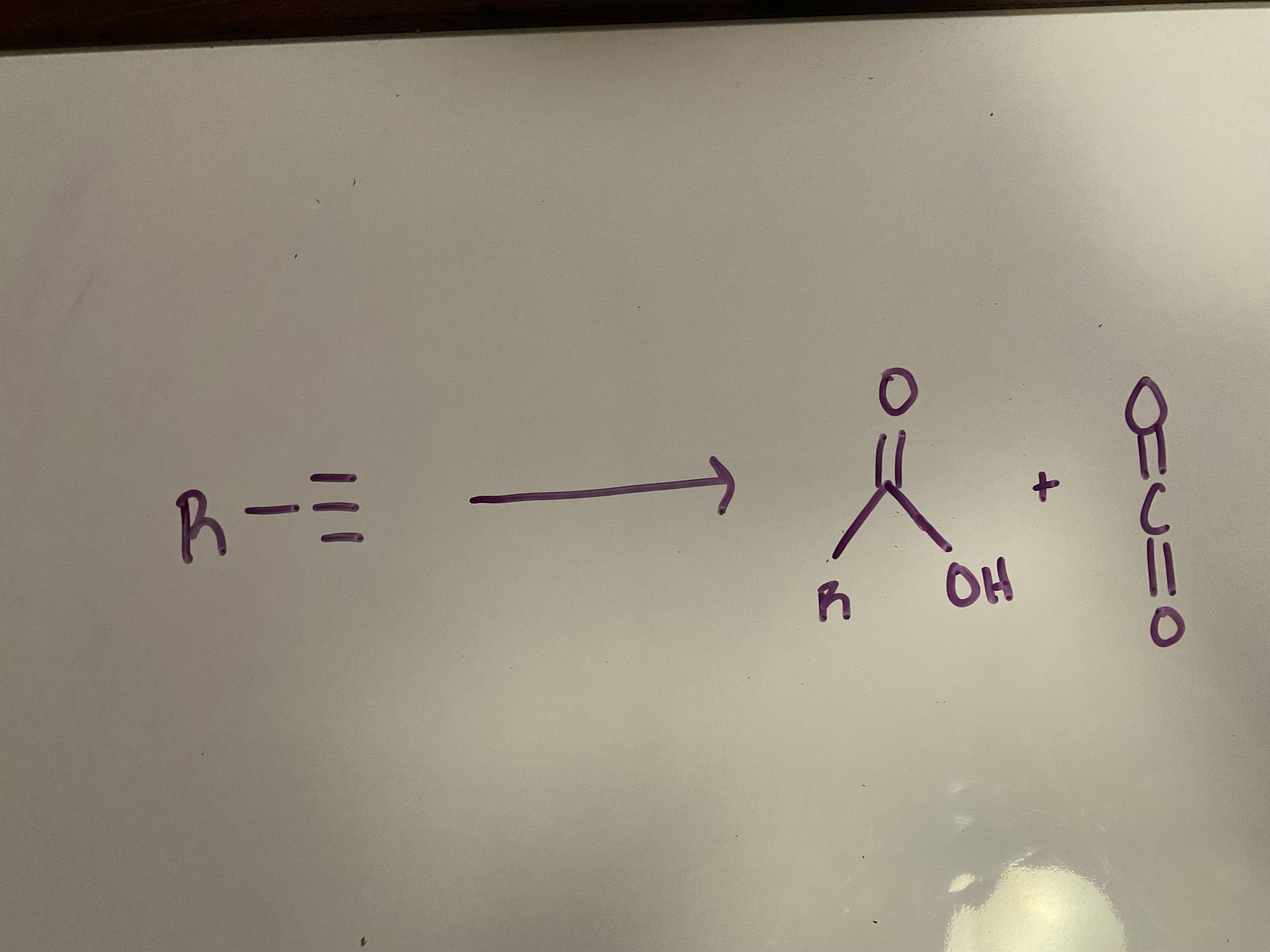
Ozoneolysis
Reagents: 1. O3 2. H2O
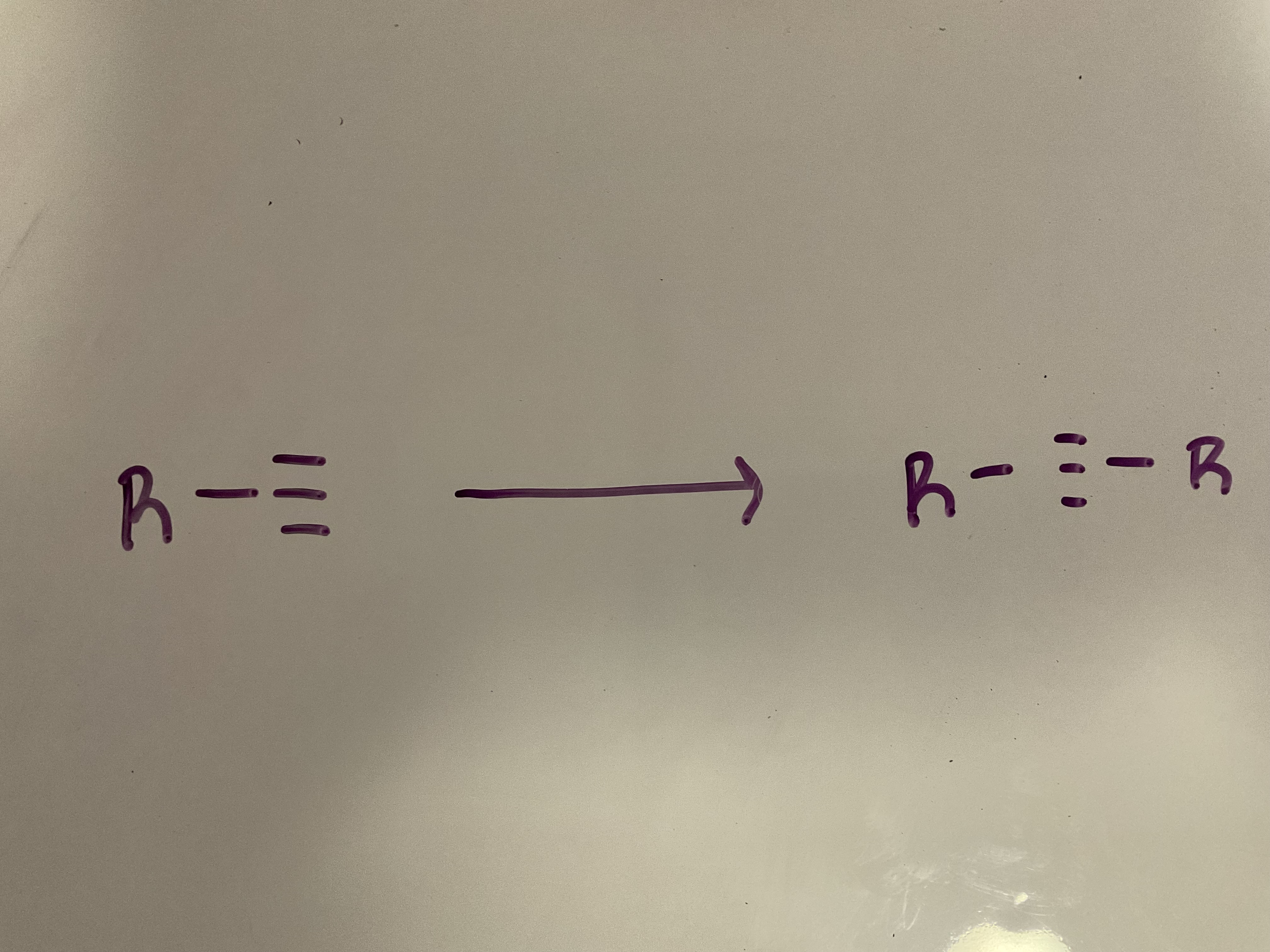
Alkylation
Reagents: 1. NaNH2 2. RX
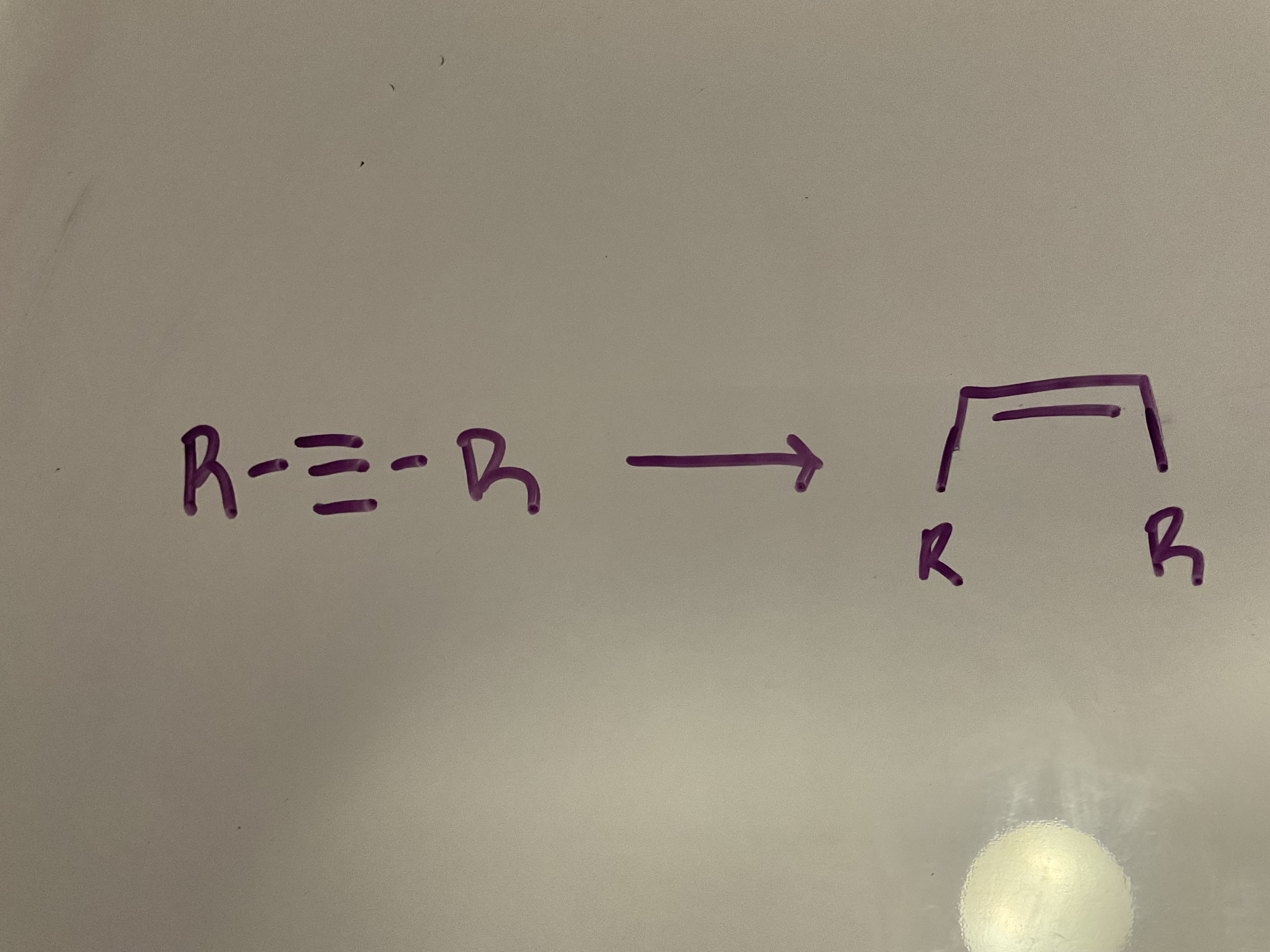
Hydrogenation
Reagents: H2, Lindlar’s catalyst
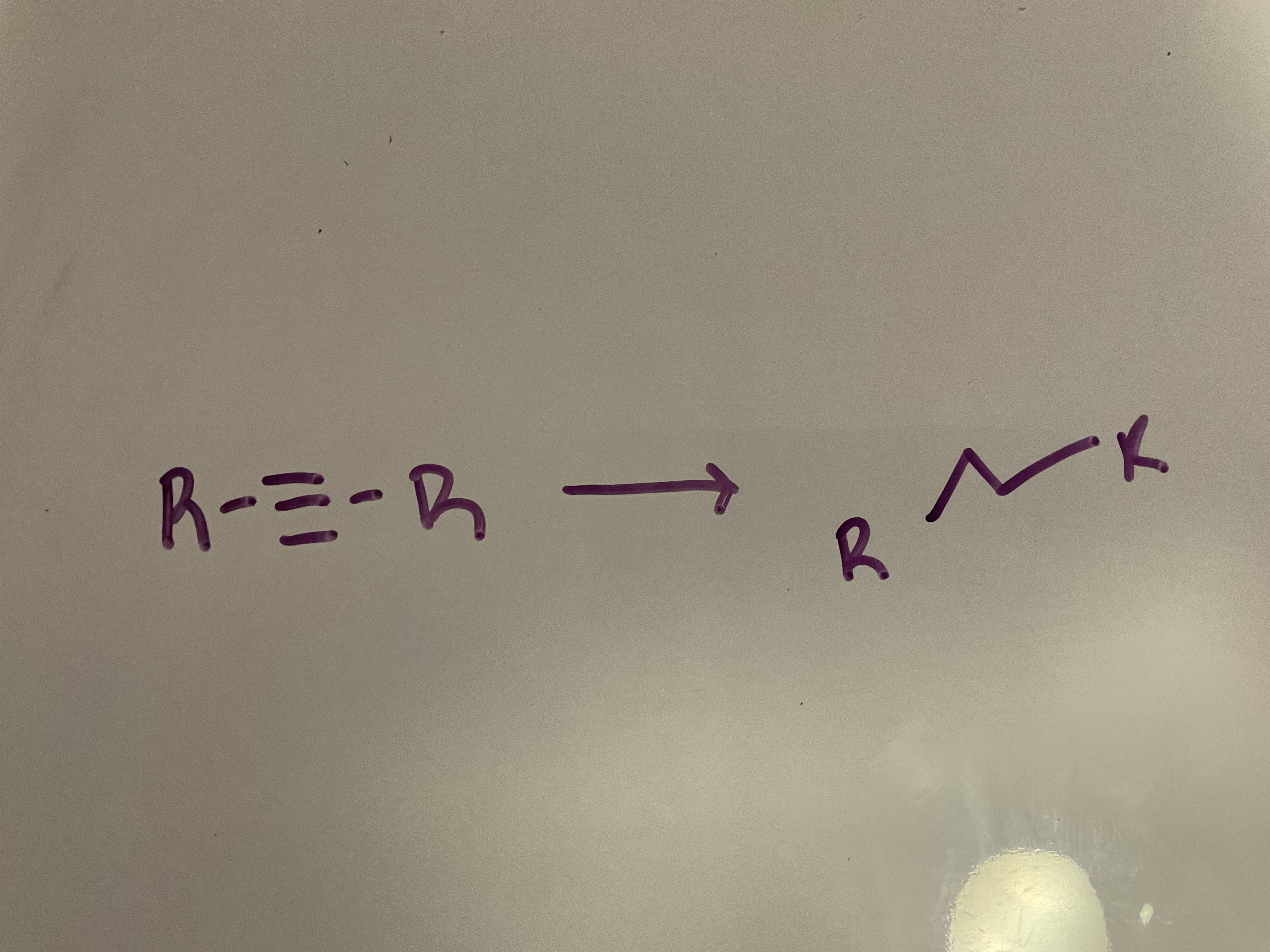
Hydrogenation
Reagents: H2, Pt
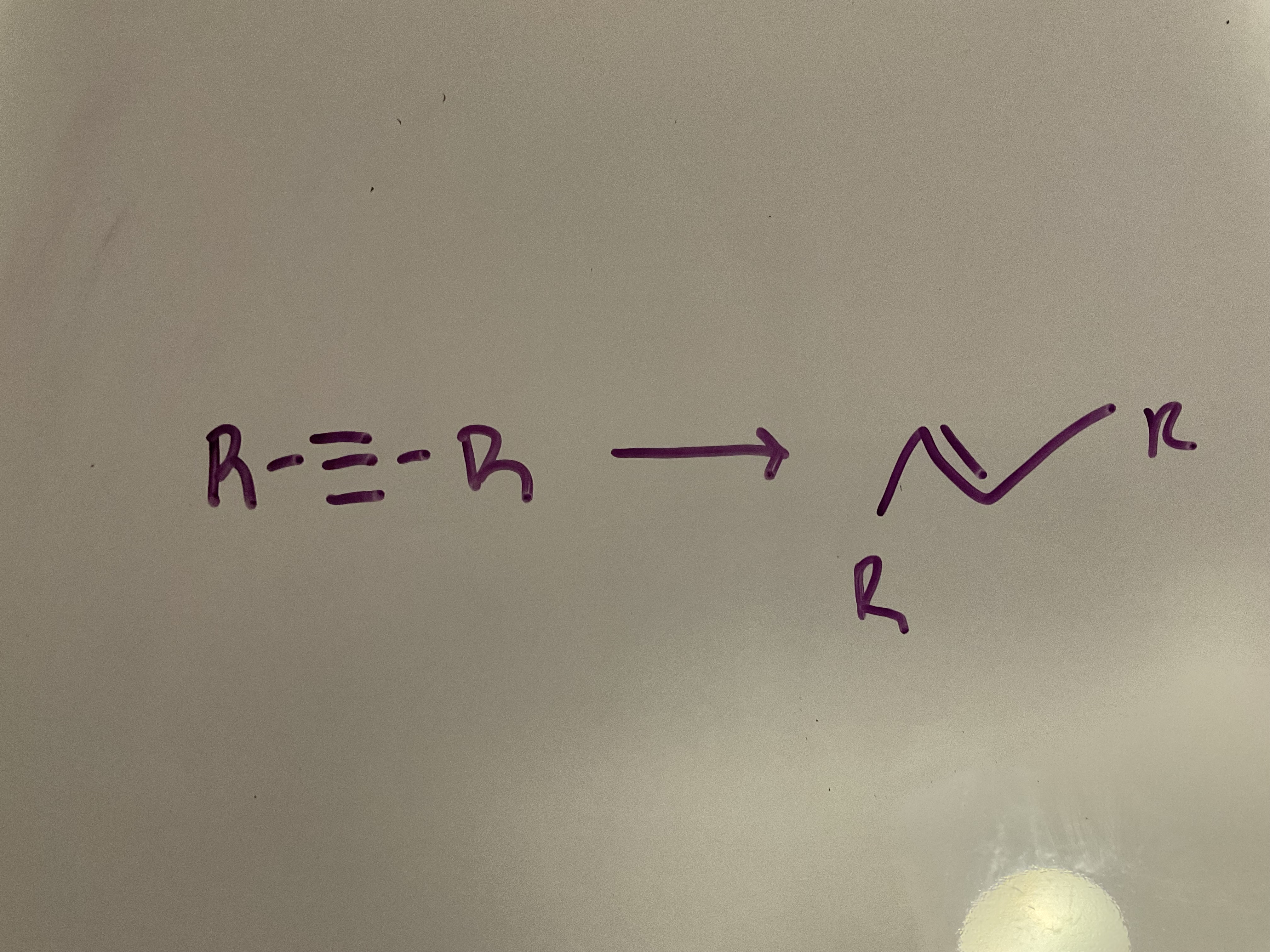
Dissolving metal reduction
Reagents: Na, NH3 (l)