Chapt 4: Organic Compounds: Cycloalkanes and their Stereochemistry
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
19 Terms
Rules in naming cycloalkanes
Find the parent (count the number of carbons in the ring and count the number in the largest substituent)
Number the substituents, and write the name
Cycloalkanes or alicyclic compounds
saturated cyclic hydrocarbons
CnH2n
Can be represented using skeletal drawings
Cycloalkanes are less
flexible than open-chain alkanes and have lesser conformational freedom in cycloalkanes
Stereoisomerism
compounds which have their atoms connected in the same order but differ in 3D orientation
Stereochemistry
term used to refer to the 3D aspects of chemical structure and reactivity
Cis-trans isomers
stereoisomers that differ in their stereochemistry about a ring or double bond
Angle strain
induced in a molecule when bond angles are forcced to deviate from the ideal 109° tetrahedral value
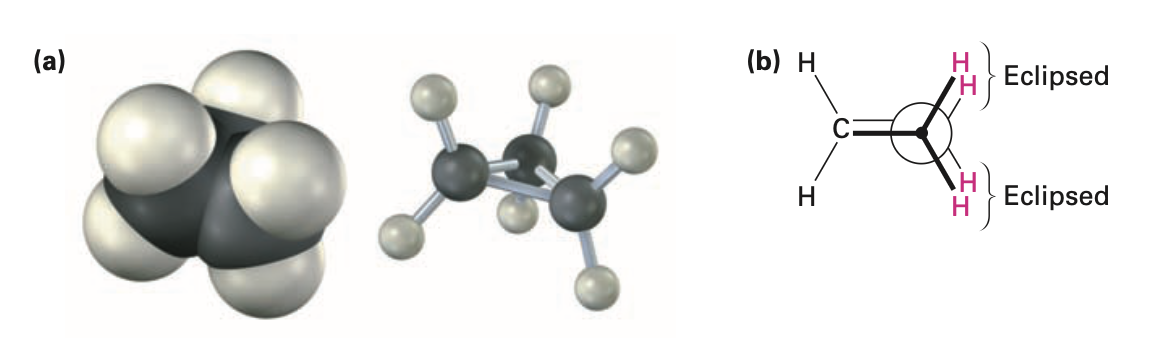
Torsional strain
caused due to eclipsing of bonds between neighboring atoms
Steric strain
caused due to repulsive interactions when atons approach each other too closely
Larger rings have many _______ than smaller rings
more possible conformations
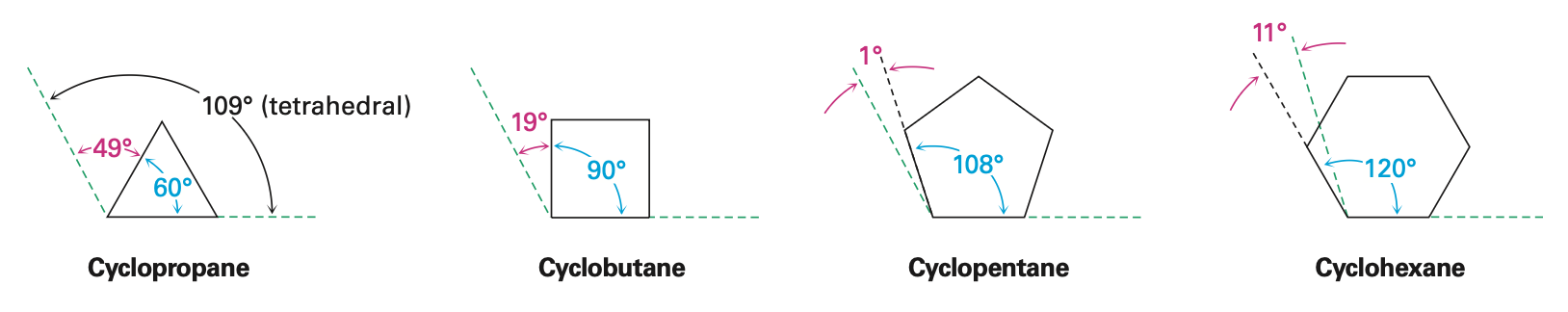
Cyclopropane
most strained of all rings due to angle strain caused by its C-C-C bond angles of 60°
has considerable torsional strain
has bent bonds
C-H bonds are eclipsed
bonds are weaker and more reactive than typical alkane bonds
Cyclobutane
Has less angle strain than cyclopropane
More torsional strain because of larger number of ring hydrogens, and their proximity to each other
Slightly bent out of plane, one carbon atom is about 25° above the plane
increases angle strain but decreases torsional strain
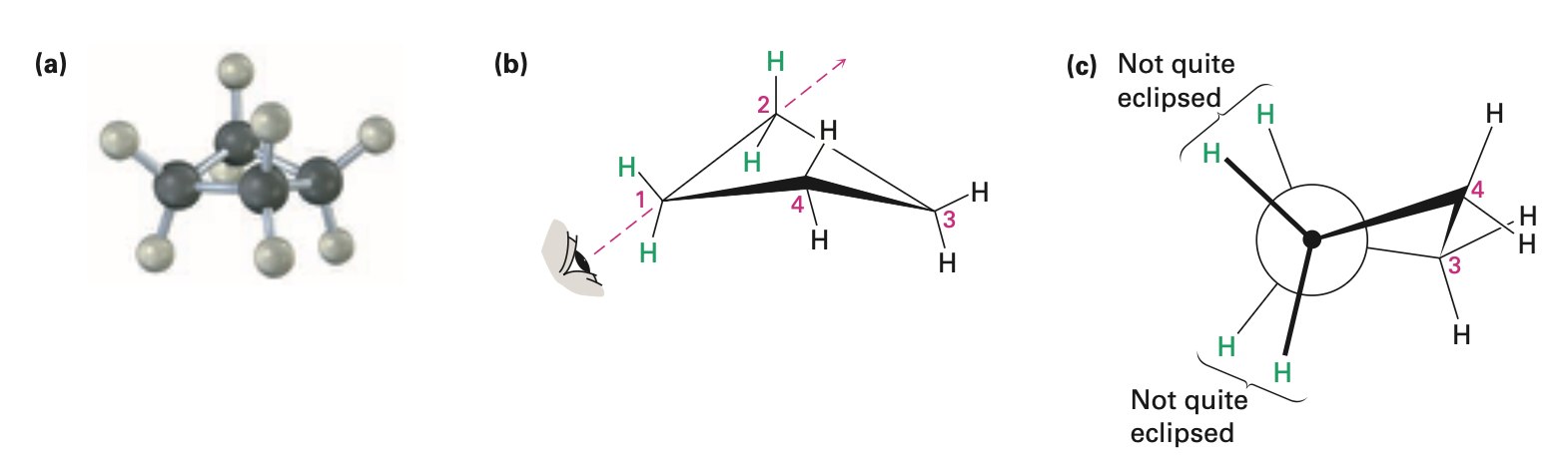
Cyclopentane
no angle strain
large torsional strain
non planar conformations strike balance between increased angle strain and decreased torsional strain
four carbon atoms are approximately in the same plane
fifth carbon atom is bent out of plane
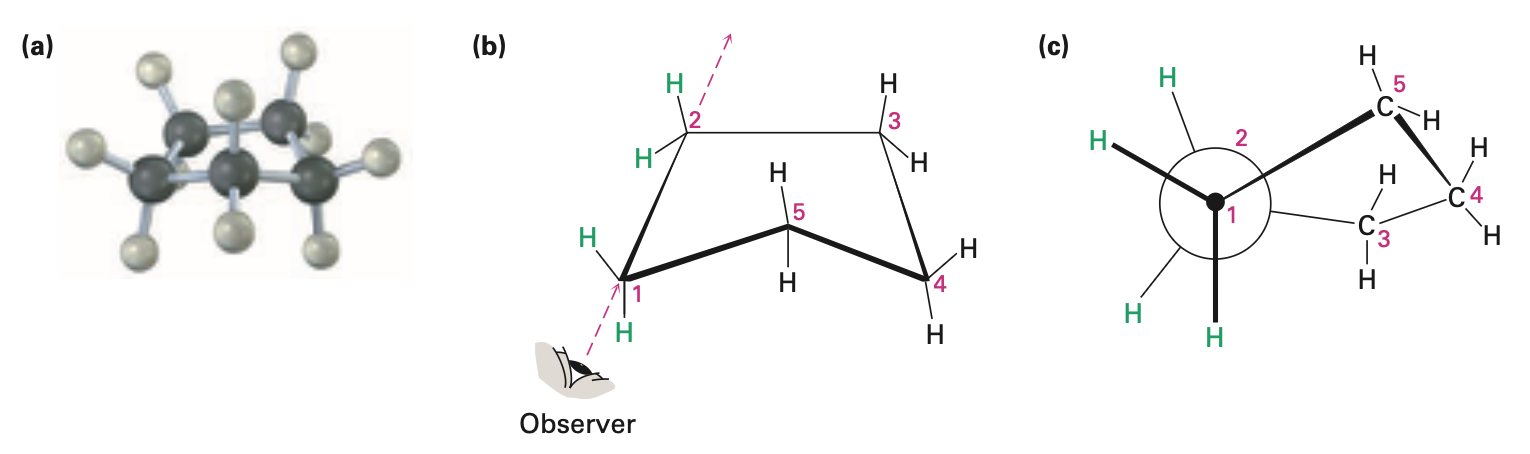
Cyclohexane
adopts chair conformation
has neither angle strain nor torsional strain
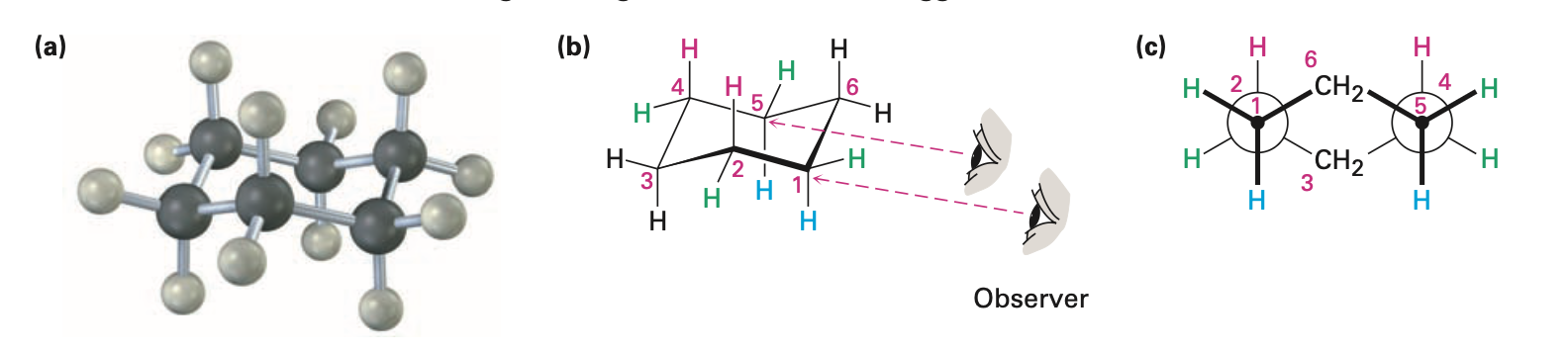
Chair conformation
has neither angle strain nor torsional strain
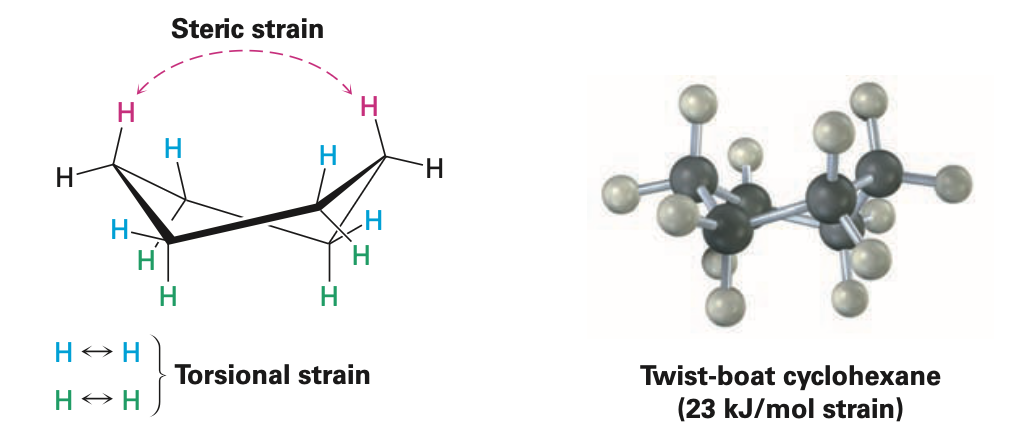
Boat cyclohexane
conformation of cyclohexane that bears a slight resemblance to a boat
no angle strain
large number of eclipsing interactions
Twist-boat conformation
conformation of cyclohexane that is somewhat more stable than a pure boat conformation
nearly free of angle strain
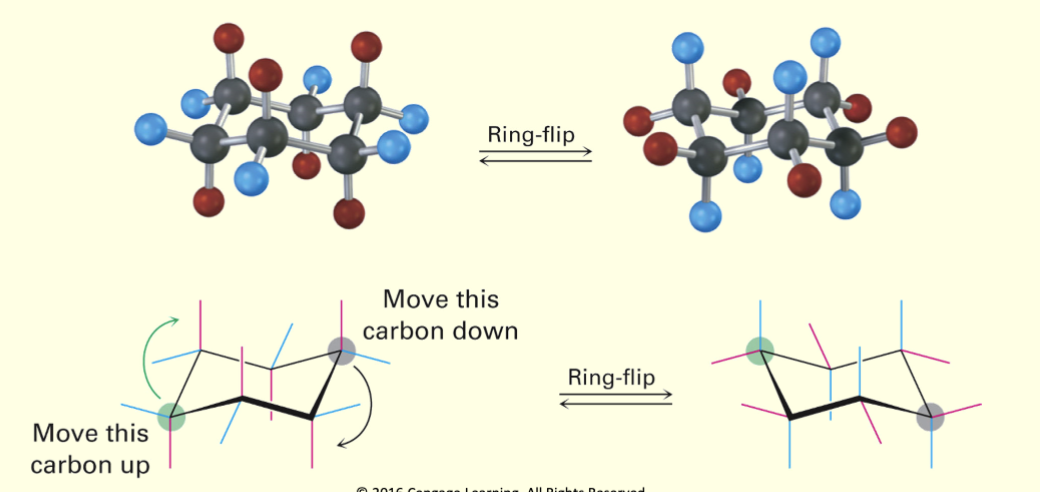
Ring-flip
Interconversion of chair conformations, resulting in the exchange of axial and equatorial positions
Gauche butane is less stable than anti butane by 3.8 kJ/mol due to
steric interference between hydrogen atoms on the two methyl groups