topic 6- group 7 - periodic table
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
Recall the colours and physical states of chlorine, bromine and
iodine at room temperature
Chlorine - yellow-green gas
Bromine - red-brown liquid
Iodine - purple solid
Describe the pattern in the physical properties of the
halogens, chlorine, bromine and iodine, and use this pattern to
predict the physical properties of other halogens
There is a trend in state change as you go down the group. This is because the melting and boiling points increase as you go down the group.
From this, you can predict that any halogens above chlorine will be gases (due to low boiling points), and any below iodine will be solids (due to high m/p)
Describe the chemical test for chlorine
When damp litmus paper is put into chlorine gas, the litmus paper is bleached and turns white.
Describe the reactions of the halogens, chlorine, bromine and
iodine, with metals to form metal halides, and use this pattern
to predict the reactions of other halogens
The pattern shows a more reactive halogen can displace a less reactive one, but a less reactive one cannot displace a more reactive one
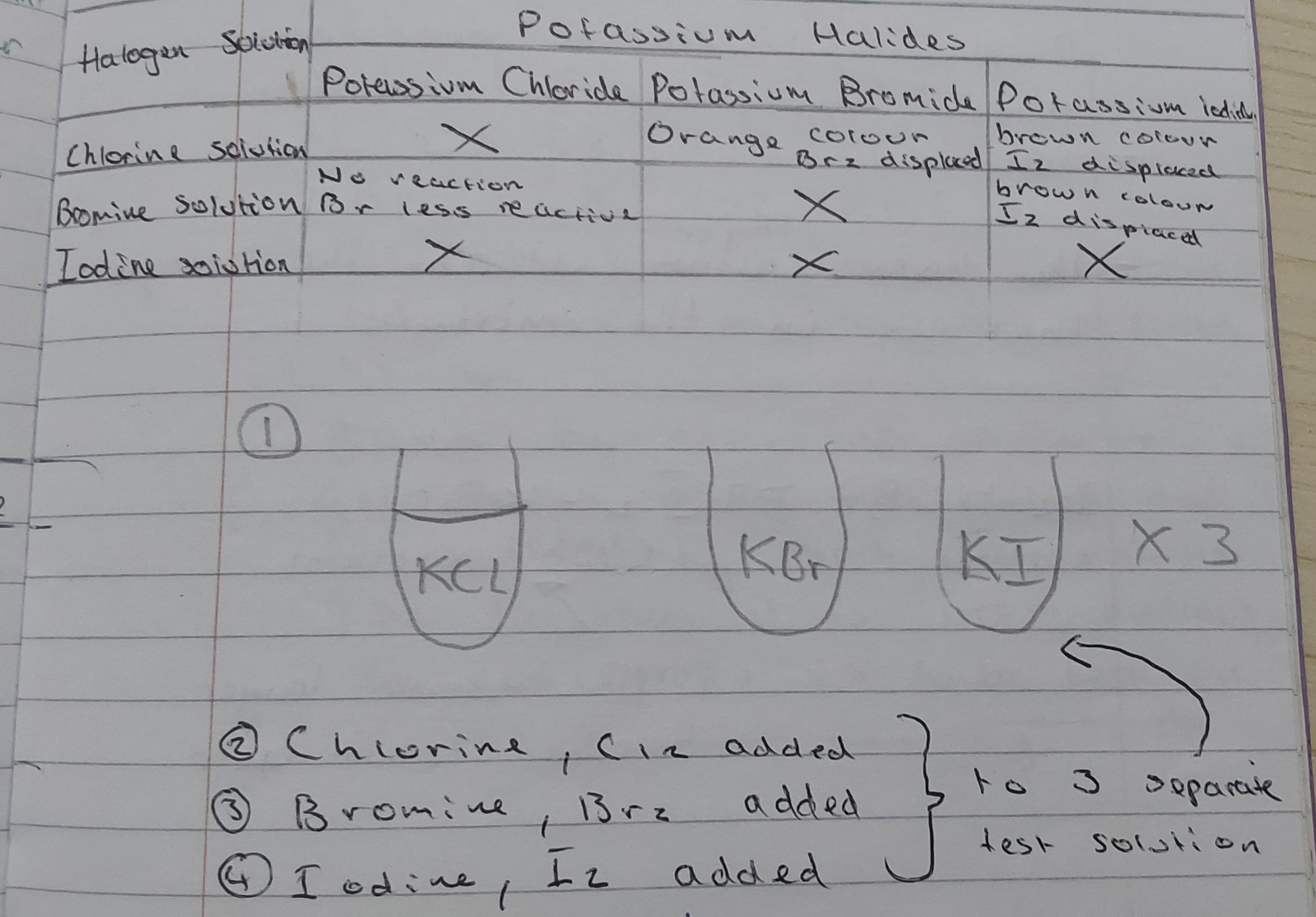
what do halogens,chlorine,bromine and iodine form
hydrogen halids which dissolve in water to form acidic solutions
Describe the relative reactivity of the halogens chlorine,
bromine and iodine, as shown by their displacement reactions
with halide ions in aqueous solution, and use this pattern to
predict the reactions of astatine

pt of previous

Explain why these displacement reactions are redox
reactions in terms of gain and loss of electrons,
identifying which of the substances are oxidised and
which are reduced
redox is when a reaction gain and loses electrons
Explain the relative reactivity of the halogens in terms of
electronic configurations
The reactivity of halogens decreases as you go down Group 7 because the atomic radius increases, making the outermost shell further from the nucleus.