AQA A level Nuclear Physics
1/67
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
68 Terms
define Isotope
Atoms of the same element with the same number of protons but different numbers of neutrons.
define Nucleon
A particle in the nucleus: either a proton or a neutron.
define Atomic Number (Z)
The number of protons in an atom's nucleus.
define Mass Number (A)
The total number of protons and neutrons in an atom's nucleus.
define Radioactive Decay
The spontaneous and random emission of radiation from an unstable nucleus.
define Alpha Decay (α) and give its equation
Emission of a helium nucleus (2 protons and 2 neutrons).
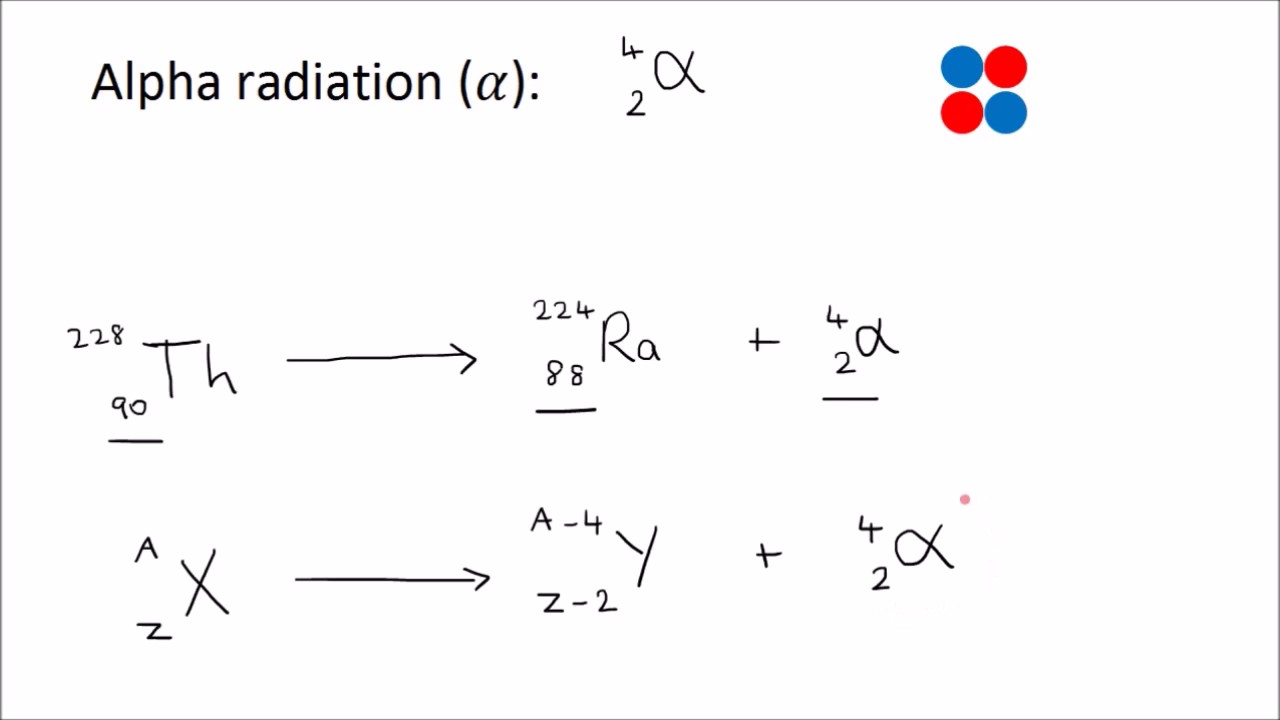
What is Beta Minus Decay (β⁻). give its equation
Neutron transforms into a proton, emitting an electron and an antineutrino.
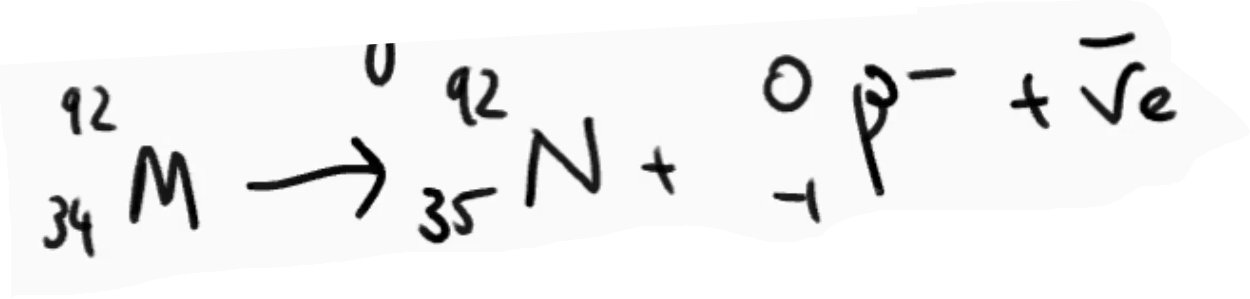
What is Beta Plus Decay (β⁺). give its equation
Proton transforms into a neutron, emitting a positron and a neutrino.
What is Gamma Decay (γ)
Emission of a high-energy photon with no change in mass or atomic number.
What is Electron Capture. give its equation
A proton in the nucleus captures an inner-shell electron and becomes a neutron.
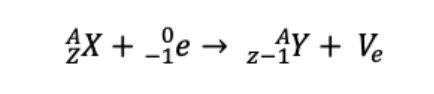
Define Activity (A). What is it measured in
The number of nuclear decays per second, measured in becquerels (Bq).
What is the Decay Constant (λ). What is it measured in
The probability per unit time that a nucleus will decay.
measured in S-1
define Half-Life (t₁/₂)
Time taken for half the radioactive nuclei in a sample to decay.
define Random
define spontaneous
random - cannot determine which and when nuclei will decay
spontaneous - is not affected by external factors
what is the Mass Defect
The difference between the total mass of the separated nucleons and the mass of the nucleus.
define Binding Energy. (draw graph as well)
The energy required to break a nucleus into its constituent nucleons.
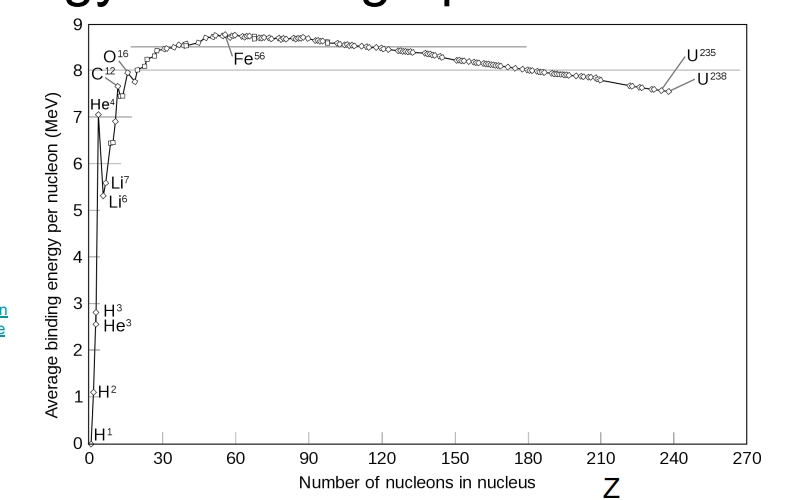
define Binding Energy per Nucleon
The binding energy divided by the number of nucleons; indicates nuclear stability.
what is Nuclear Instability / when does it happen
Occurs when a nucleus has an imbalance of neutrons and protons or is too massive.
What is Nuclear Fission. Does binding energy per nucleon increase due to fission?
Splitting of a heavy nucleus into lighter nuclei, releasing energy and neutrons.
The binding energy per nucleon increases when fission occurs
What is Nuclear Fusion. Does the new nucleus have a larger binding energy per nucleon than old nucleus?
Joining of light nuclei to form a heavier nucleus, releasing energy.
The new nucleus has a larger binding energy per nucleon than the old nuclei
Define Chain Reaction
A self-sustaining reaction where the products cause further reactions.
What is the Q-Value
The net energy change in a nuclear reaction, calculated from mass difference.
Nuclear Radius
Proportional to A^(1/3); typical values are in femtometres (1 fm = 1 x 10^-15 m).
What is the function of a moderator?
Slows down neutrons so they travel slow enough to be absorbed by the uranium.
They do this through elastic collisions between the moderator and the nucleus.
What are the properties of a moderator?
It needs to be a material that can undergo elastic collisions with neutrons
What are examples of moderators?
Water and graphite
What is the function of the control rods?
They absorb neutrons and control the rate of reaction
What are the properties of control rods?
They must be able to absorb neutrons
What is a good example of a control rod?
Boron
What is the function of the coolant?
It allows heat from the nuclear reactor to escape, which stops the reactor from overheating.
What are the properties of the coolant?
They must have a high specific heat capacity
What are some examples of coolants?
Water, carbon dioxide
What is the critical mass?
The minimum mass of fuel needed for the chain reaction to continue
Describe the Rutherford scattering experiment.
A beam of alpha particles was directed at a thin gold foil.
Occurs in a vacuum so that no collisions between air particles and alpha particles can occur.
The experiment was done in order to determine structure of an atom.
In the Rutherford scattering experiment it was observed that most of the alpha particles passed straight through. What can we infer from that?
That most of the atom is made from empty space.
What evidence was there that suggested that the nucleus had a positive charge?
Because the the nucleus repels the alpha particles and caused it to deflect from its original path, some of them even bounced back
Order Alpha, Gamma and Beta radiation starting with the most ionising?
Alpha
Beta
Gamma
Order Alpha, Gamma and Beta radiation starting with the most penetrating?
Gamma
Beta
Alpha
A sheet of paper can block which type of radiation?
Alpha radiation
When a nucleus decays through gamma radiation, how does the atomic number and mass number change?
They remain the same as the number of protons and neutrons remain the same.
Why is ionising radiation seen as dangerous?
Because it can kill or mutate cells, which could lead to mutations and lead to things such as cancer.
Which radiation is more harmful inside a human body, alpha or gamma? and why?
Alpha radiation:
because it has a high ionising power so it would damage more cells.
is also very poorly penetrating, therefore it is not be able to leave the body, whereas gamma radiation is highly penetrating.
Give an example of a real life use of Beta decay and explain why Beta is chosen for this.
Beta radiation can be used to measure the thickness of paper or aluminium foil. Alpha isn’t used as it is less penetrative and wouldn’t reach the detector on the other side of the sheet. Gamma radiation is too penetrative and would pass through everything.
Which type of radiation follows the inverse square law?
Gamma radiation.
What does the inverse square law state? whats its equation (and what does each symbol/letter represent)? give the units
The intensity is inversely proportional to the square of the distance from the source.
I = intensity (Wm-2)
K = constant
x = distance from source (m)
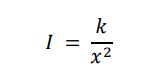
Describe an experiment which be used to show the inverse square law and gamma rays. (in detail)
Firstly measure background radiation (using Geiger Muller tube), without the gamma source in the room
Then put the gamma source at a set distance (1m) from the GM tube and measure the count rate per minute.
Record 3 measurements for each distance and take an average.
Do this for many distances going up in 10cm intervals
Take away the background radiation from each reading
Square each of the distances
Plot a graph of the count rate per minute against 1 over distance squared (1/d²)
If it is a straight line through the origin then it confirms they are directly proportional
What is background radiation?
Radiation that is constantly in the surrounding from sources such as rocks and food
What equation can you use to work out the half life of on object?
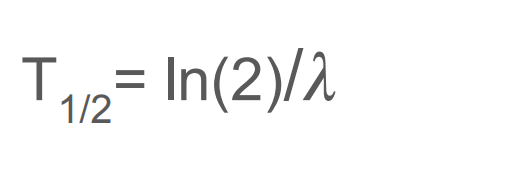
Complete the equation. 𝜆N =?
Activity (A) in Bq
True or false. Radioactive isotope decay exponentially.
True
What is the equation for the exponential decay of nuclei? (what does each symbol represent?)
N = number of nuclei at this specific time
N0 = the initial number of nuclei before decay
lambda = decay constant
t = at this specific time (the time taken) in s
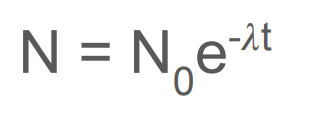
What is the equation for the exponential decay of Activity? (what does each symbol represent?)
A = activity at this time
A0 = initial activity before decay
lambda = decay constant
t = at this time (the time taken)
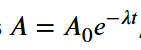
What is the equation for the exponential decay of mass? (what does each symbol represent?)
The same as the other 2 (nuclei and activity) but with respect to mass
Why is Technetium 99m useful in medicine?
Because it releases gamma radiation
It has a short half life therefore it doesn’t stay highly radioactive for long
Half life of 6 hours: long enough for it to be detected
It can also be made near to the hospital
Easy to detect outside the patient
‘Clears away’ after a few days
What does the graph of N against Z show? (also draw it)
It shows the relationship between proton number and neutron number.
The graph shows a stability curve which starts as N=Z until N value of 20.
After that, the graph curves upward and becomes steeper
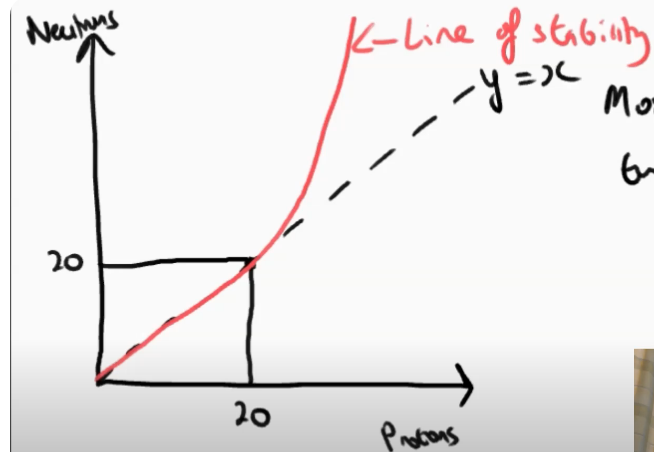
Where on the curve of N against Z does B- decay occur and why?
Above the stability line, because the nuclei found there contains too many neutrons.
Therefore when beta minus decay occurs the neutron turns into a proton and it becomes more stable.
What type of decay occurs below the stability line and why?
Beta plus decay.
As the isotopes found here often have too many protons.
Therefore when beta plus decay occurs the proton turns into neutrons.
How does the heavier nuclei often decay?
Through alpha decay.
This is because alpha decay emits a helium nucleus (2 protons and two neutrons)
therefore causing the nuclei to become less heavy and more stable.
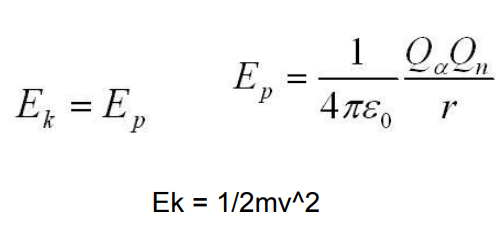
write the equations for distance of closest approach
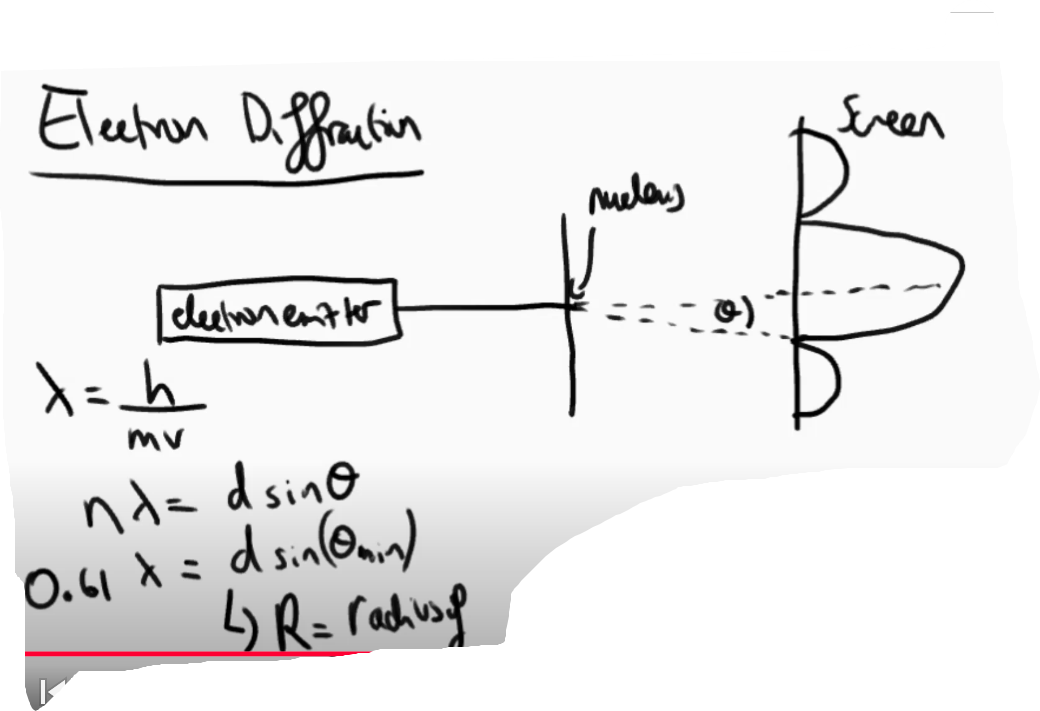
How is electron diffraction used to determine the diameter of a radius?
An electron beam is fired at a thin sheet of the desired atom.
A diffraction pattern is produced on a screen behind.
Using the angle of a minimum we can use equations to calculate the diameter.
What is the relationship / Equation between nuclear radius (R) and nucleon number (A)?
R = radius
r0 = constant (changes for diff)
A = nucleon / mass number

What equation is used to convert mass to its energy equivalent? (units and what does each represent)
E = energy (J)
m = mass (kg)
C = speed of light (ms-1)
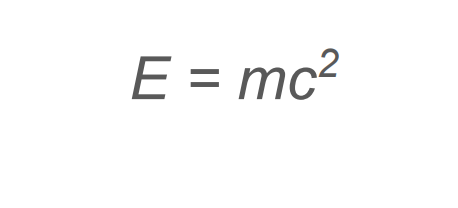
Why is there a mass defect?
Because energy is needed to bring the constituent parts of a nucleus together, therefore the mass equivalent of the energy is lost and the total mass decreases.
Why is it difficult to make fusion occur on earth?
There is a large repulsion between the two positively charged nuclei, therefore a lot of energy is required to overcome the repulsion and fuse them together.
It is hard to get a material that can withstand the heat and be cost effective.
How is fission used in nuclear reactors?
Rods of uranium-235 absorb neutrons and become unstable and then split into two daughter nuclei.
It also releases 2-3 more neutrons. These then go on to be reabsorbed by another uranium-235.
Which waste products from a nuclear reactor cause the highest risk?
Spent fuel rods.
How is high-level waste disposed of?
They are first stored in cooling ponds
Then they are put in sealed steel containers and put deep underwater
What is low level waste contained in?
They are sealed in containers and put underground until it is safe again.