MICR 270 Mod 4 - adverse reactions & immune defects
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
Immunodeficiency
an immunodeficiency is a disorder or condition where the immune system has reduced function or is absent and can be traced to failure of one or more parts of the immune system
Primary immunodeficiency
primary immunodeficiencies are congenital (present from birth) and derive from a genetic or developmental defect leading to abnormal maturation of the immune system
primary immunodeficiencies may be associated with defects in the innate or adaptive immune systems
primary immunodeficiencies are rare as in most cases, the fetus will not survive the defect
secondary immunodeficiency
secondary immunodeficiencies are acquired and result from environmental factors affecting and compromising the immune system
causes of secondary immunodeficiency include:
undergoing chemotherapy treatment
taking immunosuppressive medication
contracting a chronic infection
ie. HIV/AIDS
developing cancer
ie. leukemia, multiple myeloma, lymphoma
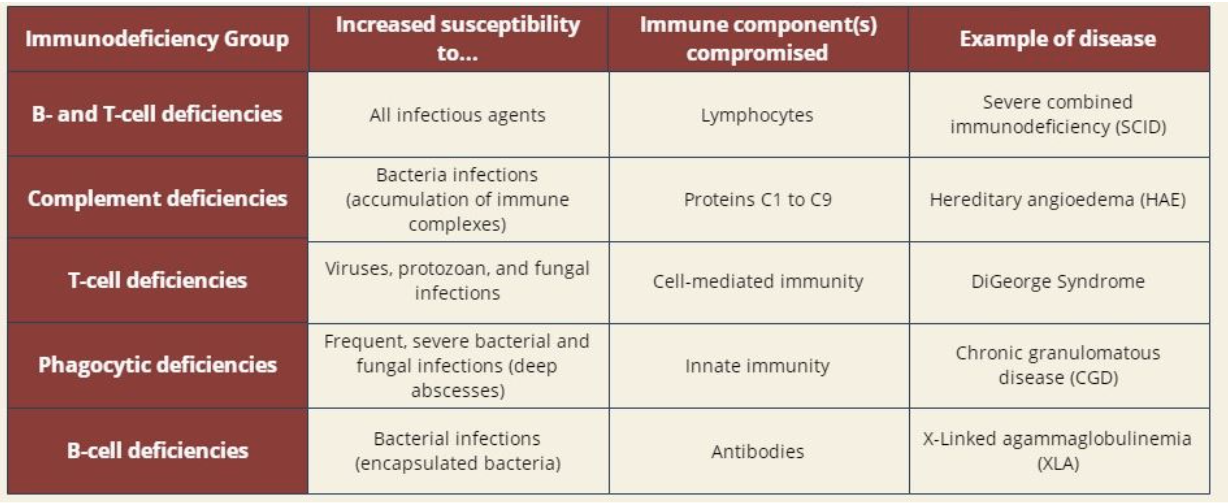
B-cell primary immunodeficiency
B cell deficiencies are characterized by dysfunctional B lymphocytes or a decrease in their prevalence
B lymphocytes are the key cells of humoral immunity as they produce large quantities of antibodies
a deficiency in B cell development results in an increased susceptibility to infection, especially by incapsulated bacteria
first symptoms generally appear around 7-9 months old, as the IgG immunoglobulins transferred from the mother begin to decrease, and the infant does no synthesize enough antibodies to compensate
B-cell primary deficiency example: X-Linked Agammaglobulinemia (XLA)
XLA is a rare genetic disorder
it is X-linked recessive so occurs almost exclusively in males
patients with the disease do not develop mature B cells and as a result have extremely low levels of IgG and lack all other immunoglobulins
XLA patients are extremely susceptible to bacterial infections, but their susceptibility to viral and fungal infections remains unchanged
this is because their cell-mediated immune responses remain normal
T-cell primary immunodeficiency
T-cell deficiencies are characterized by dysfunctional T lymphocytes or a decrease in their prevalence
T lymphocytes are the key cells of cell mediated immunity as they kill infected or abnormal cells
a deficiency in T cell development results in increased susceptibility to viruses, protozoans, and fungi
T cell deficiencies are often characterized by frequent infections beginning 3-4 months after birth
common examples of infections are pneumonia and candidiasis
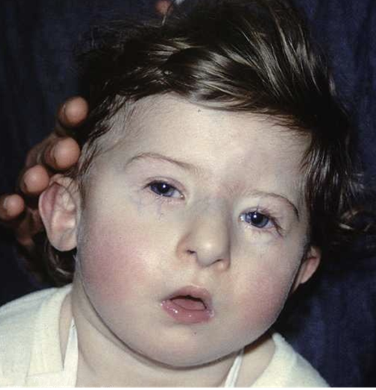
T cell primary immunodeficiency example: DiGeorge Syndrome
DiGeorge syndrome is a complex disease caused by the deletion of a small segment of chromosome 22
DiGeorge Syndrome patients have an absent or underdeveloped thymus, which results in the absence of mature T cells
in addition to T cell abnormalities, abnormalities in the heart, face, and palate are commonly observed as well as learning disabilities
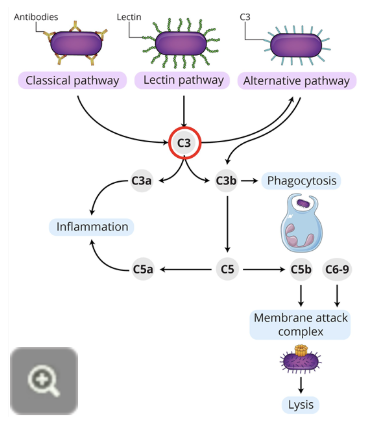
Complement system primary immunodeficiency
the complement system performs multiple functions and involves the intricate regulation of nine components
genetic deficiencies have been described for each of these complement components
patients with complement deficiencies are prone to frequent severe bacterial infections and complications arising from inability to clear immune complexes
in particular, those with C3 (circled in diagram) deficiencies display the most severe symptoms, reflective of the central role played by this component in complement activities
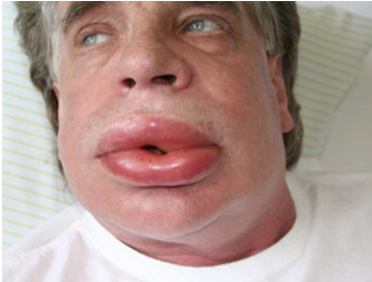
complement system primary immunodeficiency example: Hereditary Angioedema (HAE)
complement deficiencies can also arise from deficiencies in proteins that regulate complement pathways
patients with HAE lack a regulator of C1
symptoms of HAE include swelling of the face, lips, larynx, or GI tract
the swelling of the larynx or GI tract are of particular concern as this can lead to suffocation or acute abdominal pain
Phagocytic primary immunodeficiency
the phagocytic process is an important part of innate immunity, as extracellular pathogens are engulfed and destroyed within a phagocyte
phagocytic deficiencies can appear at various stages of this process
in patients with defective phagocytes, bacterial and fungal infections are usually frequent and severe, often causing deep abscesses
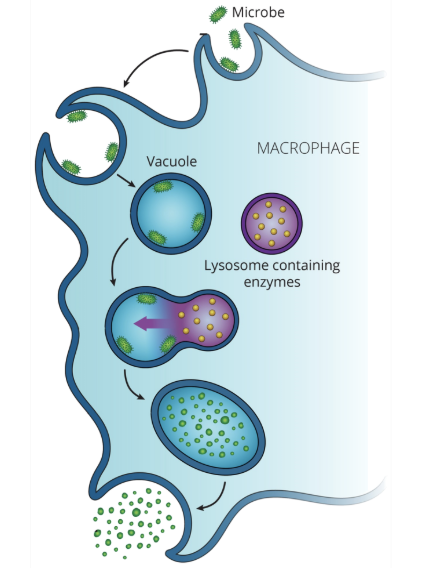
phagocytic primary immunodeficiency example: chronic granulomatous disease (CGD)
CGD is a rare inherited disease where the body’s phagocytes do not make the chemicals needed to kill phagocytosed bacteria
CGD derives its name from the tendency of patients with this disease to form non-malignant granulomas (small, nodular aggregations of immune cells) in order to attempt to separate foreign materials from the rest of the body
combined B cell and T cell primary immunideficiency
individuals with combined B cell and T cell deficiencies have dysfunctional and/or low numbers of lymphocytes
as a result, bot the humoral and cell mediated responses of the adaptive immune system are compromised
this deficiency is characterized by little to no resistance to infection, thus pathogens that cause mild disease in the average human (ie. chickenpox) may be life threatening
patients with combined B cell and T cell deficiencies often suffer fatal infections within the first year of life
combined B cell and T cell immunodeficiency example: severe combined inherited immunodeficiency (SCID)
SCID is a classic example of combined B cell and T cell immunodeficiency
David Vetter, dubbed “Bubble Boy” by the media, was raised in a sterile room for 12 years
because of this, he never encountered any infections
a spacesuit invented by NASA allowed him to venture a short distance from the room
David passed away in 1984 after a bone marrow transplant intended to treat his disease, which contained an unexpected infectious agent
secondary immunodeficiency: HIV/AIDS (acquired immunodeficiency syndrome)
secondary immunodeficiencies are not as easily classified as primary deficiencies
the most well known and well studied secondary immunodeficiency disease is HIV/AIDS
Acquired: individuals do not inherit this type of disease, which is a major difference between AIDS and the previously discussed primary immunodeficiency diseases
Immunodeficiency: the one disease characteristic AIDS victims have in common is the breakdown of the immune system
Syndrome: the plethora of rare but ravaging diseases that take advantage of the body’s collapsed defences
AIDS is the final stage following an acute HIV infection
many AIDS patients die from opportunistic infections as their immune system is compromised and unable to effectively protect and defend the body
primary mode of transmission of HIV
North America: sexual intercourse
Eastern Europe and Central Asia: use of non-sterile injecting drug paraphernalia
Sub-Saharan Africa: heterosexual sex with a contaminant epidemic in children through vertical transmission (mother-to-child)
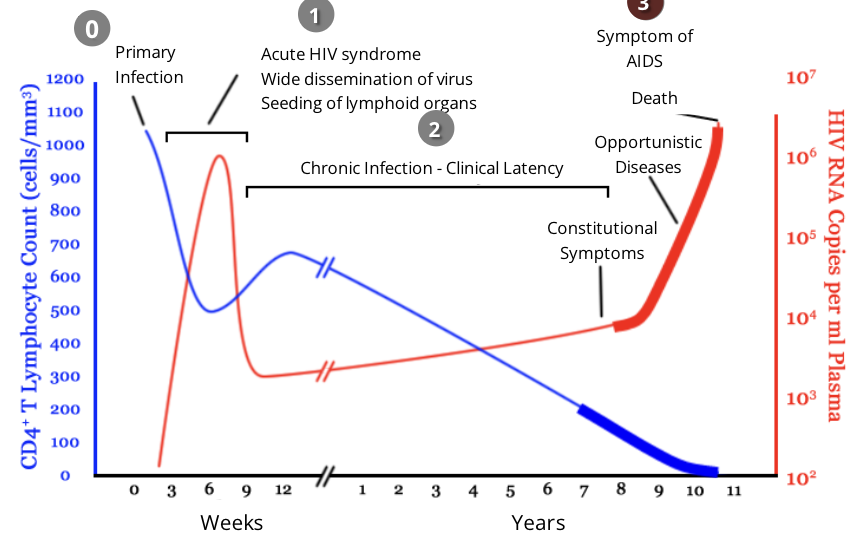
HIV and the immune response: stage 0, primary infection
upon infection with HIV, most people will mount an effective immune response to the virus for the first couple weeks
over time, this response will prove ineffective through the various stages of the disease, as the HIV virus compromises the individual’s immune system
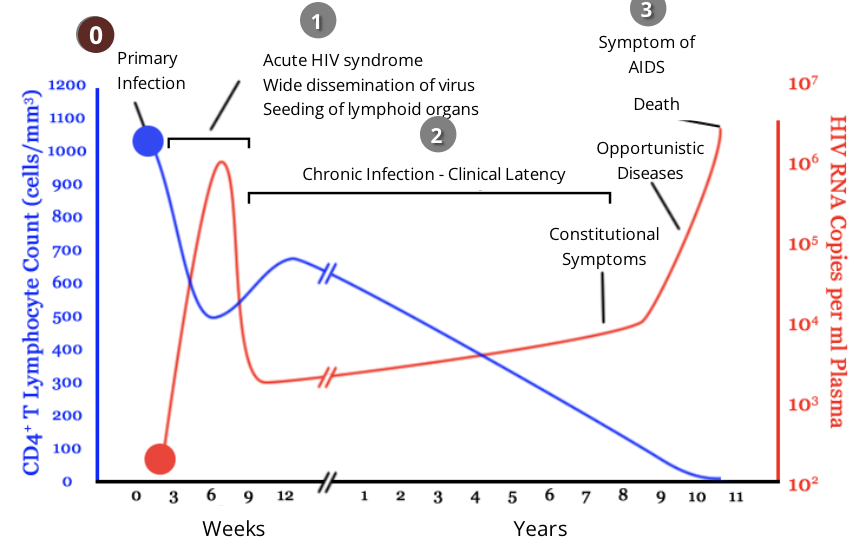
HIV and the immune response: stage 1, acute infection
during acute infection, HIV targets and infects cells with CD4 on their surface, including CD4 helper T cells
viral infection causes a drastic decrease in the level of CD4 helper T cells while the level of viruses in the blood increases
within 2-4 weeks after primary exposure to HIV, some people will experience flu-like symptoms including fever, rash, headache
the level of HIV in the blood is very high during the acute infection phase, which greatly increases the risk of HIV transmission
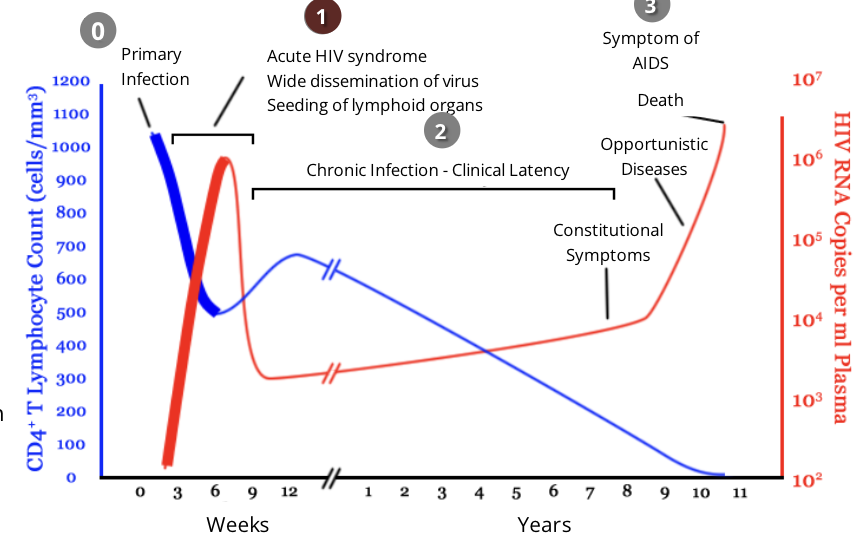
HIV and the immune response: stage 2, chronic infection
during this stage, HIV continues to multiply in the body at a steady rate
people with chronic HIV infection often do not experience any HIV related symptoms, however transmission is still possible
Anti-HIV antibodies are detectable during this phase of infection
however, HIV can begin to evade the immune response that is present by changing their antigens through high mutation rates
the length of this phase can vary but is generally 8-10 years
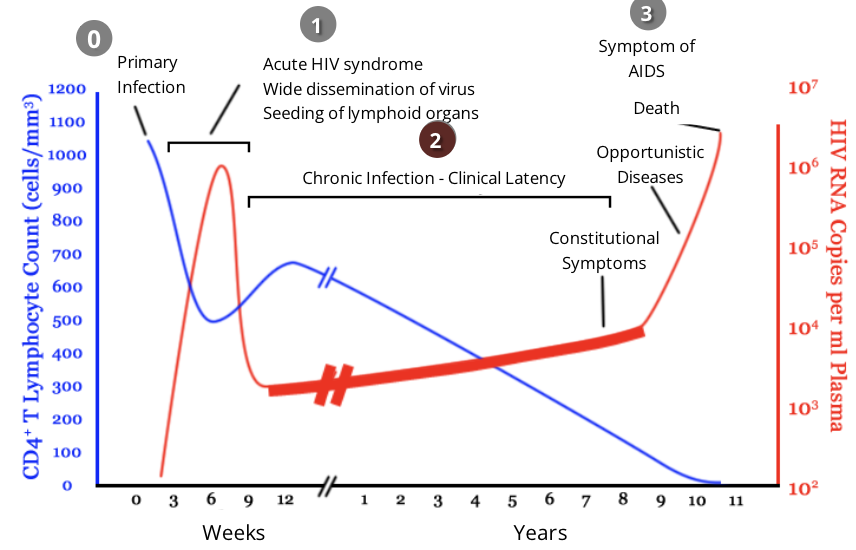
HIV and the immune response: stage 3, AIDS
throughout clinical latency, CD4 helper T cells get ‘exhausted’ and depleted while constantly fighting a chronic HIV infection
HIV patients are diagnosed with AIDS if they have a CD4 helper T cell level of less than 200 cells/mm³
after this point, viral load drastically increases as the virus continues to acquire mutations that allow it to further avoid immune defences
as the immune system is severely weakened, patients become extremely susceptible to opportunistic infections
in the absence of treatment, AIDS patients typically survive about 3 years
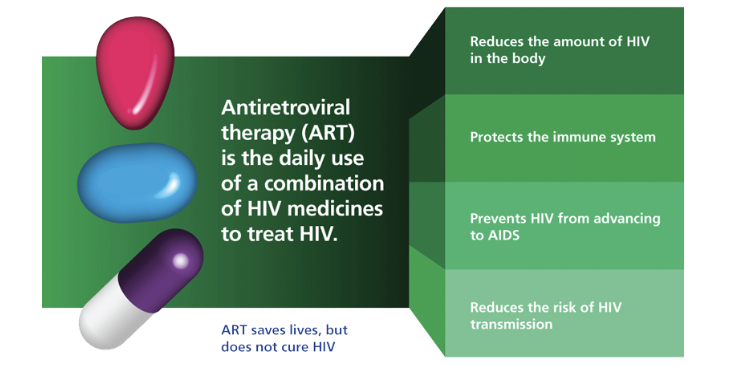
Antiretroviral Therapy (ART) for HIV patients
the first ART was approved in 1987. These drugs do not kill or cure the human immunodeficiency virus, but can prevent it from replicating
since then, combination retroviral therapy (aka antiretroviral therapy ART or highly active antiretroviral therapy HAART), has been introduced
this therapy utilizes a panel of antiretroviral drugs in different combinations to prevent drug resistance by the rapidly mutating virus
this treatment method has led to staggering declines in the rates of AIDS and AIDS-associated deaths
HAART maintains the function of the immune system, and prevents opportunistic infections that often lead to death
summary of primary immunodeficiencies
screening techniques for immunodeficiencies: Complete Blood Counts (CBCs)
CBCs show how many of each cell type are present in a small sample of patients’ blood
these numbers are compared to a reference range of values commonly found in healthy people
this technique is used to highlight any severe defects in the blood that could potentially be caused by an immunodeficiency
CBCs are readily available to physicians and are often used to guide the use of more detailed tests of specific cell types
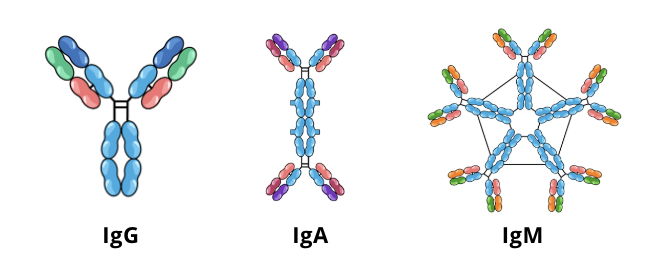
screening techniques for immunodeficiencies: Quantitative Serum Immunoglobulin
quantitative serum immunoglobulin tests measure the levels of IgG, IgA, and IgM in a patient’s blood serum and compare them to a control
if the levels of antibodies in the blood are lower than normal (hypogammaglobulinemia), this could be an indication of a humoral immunodeficiency
further testing such as complete blood counts and urine protein electrophoresis (a screening test to evaluate the amount of certain proteins in urine) can be used to pinpoint the source of the hypogammaglobulinemia
autoimmunity & autoimmune disease
Autoimmunity
in some circumstances, the immune system initiates a reaction in response to its own cells
this reaction to self is what is called autoimmunity
Autoimmune disease
failure of an organism to distinguish self from non self causes the immune system to initiate a response against its own cells and tissues
any disease that results from such an aberrant immune response is termed an autoimmune disease
autoimmune disease by the numbers
autoimmune disease affects 5-7% of the human population
autoimmune diseases more commonly affect females than males
approx 78% of individuals infected with an autoimmune disease are women
organ-specific autoimmune diseases
organ specific autoimmune diseases involve an immune response that is directed to an antigen that is unique to a single organ or gland
as a result, the disease manifestations are largely limited to the specific organ
Target organs:
the most common organs of the body affected by autoimmune diseases are:
thyroid gland
adrenal glands
stomach
pancreas
Example of organ-specific autoimmune disease:
Graves disease (leads to hyperactivity of the thyroid gland)
organ-specific autoimmune disease: graves disease
Normal TSH function
thyroid-stimulating hormone (TSH) is produced by the pituitary gland and is crucial for regulating the production of thyroid hormones
binding of TSH by receptors on thyroid cells stimulates the production of thyroid hormones, which control many aspects of metabolism
negative feedback by thyroid hormones allows TSH production from the pituitary gland to be moderated
Graves Disease
patients with graves disease produce autoantibodies to the receptor for TSH
these autoantibodies continuously engage TSH receptors, but unlike TSH, cannot be moderated
this results in unregulated overproduction of thyroid hormones, leading to metabolic dysfunction
Causes and Symptoms
the exact cause of graves disease is unknown but is thought to be a result of both genetic and environmental factors
overstimulation of thyroid cells can lead to enlargement of the thyroid gland, a condition referred to as a goiter
the metabolic dysfunction caused by graves disease can result in weight loss, rapid heart beat, poor regulation of body temperature, muscle weakness, and irritability
Systemic autoimmune diseases
in a systemic autoimmune disease, the immune response is directed towards a broad range of antigens that are characteristic of a number of organs and tissues
Example of a systemic autoimmune disease
rheumatoid arthritis is a common autoimmune disorder that typically presents a s chronic inflammation of joints, however other organ systems can also be affected
Rheumatoid Arthritis
most commonly observed in women aged 40-60
many patients with rheumatoid arthritis produce autoantibodies, most commonly IgM, to portions of the Fc receptor of IgG, which are referred to as rheumatoid factors
rheumatoid factors bind to circulating IgG forming immune complexes that become deposited within joints
these deposits can activate the complement cascade, leading to prolonged inflammation and ultimately joint tissue damage
Immunosuppression
autoimmune diseases are typically treated using a class of drugs called immunosuppressants
these drugs suppress or reduce the strength of the body’s immune response
in an ideal world, treatment of autoimmune diseases would involve reducing only the erroneous autoimmune response while leaving the rest of the immune system intact
however, scientists have yet to discover a way in which this can be accomplished
Immunosuppression and organ transplants
in addition to treating autoimmune diseases, immunosuppressant drugs are commonly administered to individuals who have undergone an organ transplant
after transplantation, the body recognizes the new organ as a foreign object and the immune system will initiate a response against it
immunosuppressant drugs are therefore used to reduce the risk of rejection by inhibiting the immune response and allowing the organ to remain healthy in its new environment
because of their compromised immune system, its important to individuals on immunosuppressants to remain healthy and avoid infection - as their immune system bay not be capable of fighting off foreign microbes
Classes of immunosuppressive drugs
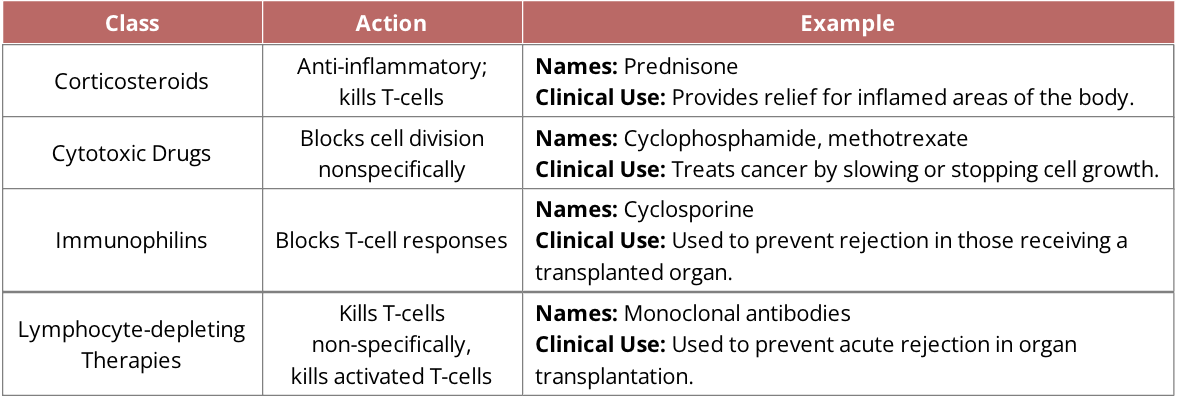
mechanism of immunosuppressive drugs
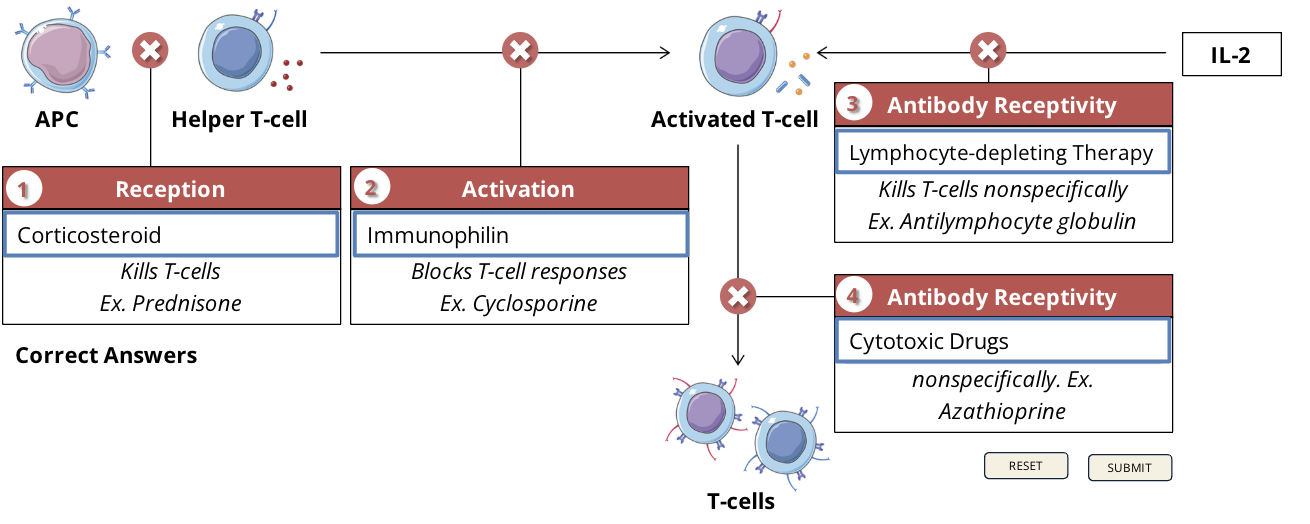
downsides of immunosuppressive drugs
Immunophilin: cyclosporine
nephrotoxicity, hypertension, hirsutism, hypertrichosis, gingival hyperplasia
Cytotoxic drugs: cyclophosphamide
nausea, vomiting, loss of appetite, stomach ache, diarrhea, darkening of skin/nails
Cytotoxic drugs: methotrexate
nausea, vomiting, hair loss, tiredness, dizziness, chills, headache, mouth sores, sores in lungs, increased risk of skin infection, sun sensitivity, rash, stuffy or runny nose and sore throat, low blood cell levels
Corticosteroids: prednisone
osteoporosis, hirsutism, hypertrichosis, diabetogenic
impact of immunosuppression on the host
Latent Infections
individuals that are on immunosuppressive therapy have an increased risk of reactivation of pathogens that are usually associated with latent infections (infections that are inactive, hidden, or dormant)
most common pathogens include tuberculosis TB, herpes simplex virus HSV1/2, cytomegalovirus CMV, epstein barr virus EBV, varicella zoster virus VZV
Opportunistic Infections
opportunistic infections commonly occur when there is reactivation of a pathogen that is already present in the host
these infections can also result when a pathogen is picked up from the environment, but the blunted immune response of the host is unable to combat the pathogen
opportunistic infections can arise from bacteria, viruses, parasites, or fungi
Fungal opportunistic infections
Name: pneumonocytis jiroveci pheumonia
common name: PCP
infects: pneumonia of lungs
Name: cryptococcosis
common name: cryptococcal disease
infects: lungs, which may also spread to the brain
Name: candidiasis
common name: thrush
infects: mouth, throat, vagina
Name: aspergillosis
common name: N/A
infects: lungs
Bacterial opportunistic infections
Name: tuberculosis
common name: consumption
infects: lungs
Name: myobacterium avium complex
common name: MAC
infects: lungs, lymph nodes, or entire body depending on site of infection
parasitic opportunistic infections
Name: toxoplasmosis
common name: N/A
infects: skeletal muscle, myocardium, brain, eyes
Viral opportunistic infections
Name: cytomegalovirus
common name: CMV
infects: eyes, brain, or other internal organs
Name: Herpes simplex virus (HSV)
common name: herpes
infects: skin, mouth, lips, eyes, genitals
Name: varicella zoster virus (VZV)
common name: chickenpox
infects: skin, or in more extreme cases, internal organs
Name: mononucleosis
common name: epstein-barr virus or kissing disease
infects: lymph nodes, throat, salivary glands, liver, spleen, blood
Classifications of Hypersensitivities
Type I: immediate/anaphylaxis
allergic reactions ie. food allergies
Type II: cytotoxic
Blood diseases ie.
transfusion reactions
hemolytic disease of the newborn
Type III: immune complex-mediated
contribute to development of autoimmune diseases ie.
systemic lupus erythematosus
rheumatoid arthritis
Type IV: delayed-type
skin reactions ie. contact dermatitis
Type I hypersensitivity (immediate/anaphylaxis)
Mediators
allergens
normally a harmless substance, an allergen produces an abnormal immune response called an allergic reaction. In humans there are 8 major food allergens: milk, eggs, fish, shellfish, treenuts, peanuts, wheat, soya
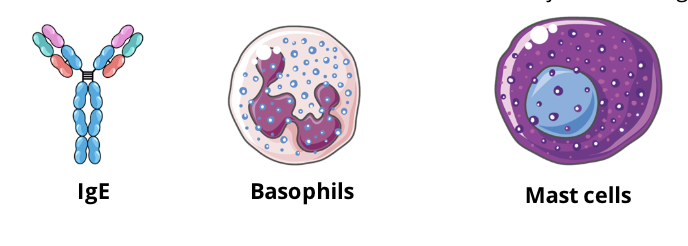
IgE, basophils, mast cells
Mechanism of Reaction
Primary exposure to an allergen
the allergen induces a humoral immune response wherin plasma cells secrete an excessive amount of IgE antibodies which bind to mast cells and basophils
Secondary exposure to the same allergen
membrane bound IgE cross-links with the allergen which initiates the degranulation (release of granules) of basophils and mast cells, releasing vasoactive mediators causing vasodilation and smooth muscle contraction
Reaction time
Type I hypersensitivity reactions can be immediate (minutes - anaphylactic reactions) and lead to death in as little as 15 minutes
rare type I reactions can take longer (developing after 24 hours), but most occur soon after exposure
Clinical manifestation
allergic rhinitis
generalized irritation of the nose when the immune system overreacts to allergens in the air
atopic determatitis (eczema)
a condition where an individual develops skin eruptions accompanied by redness
asthma
a respiratory condition in which the airways narrow, swell, and produce extra mucus
hives (urticaria)
a rash of itchy round welts on the skin that may also burn, sting, or swell
Type II Hypersensitivity (cytotoxic)
Mediators
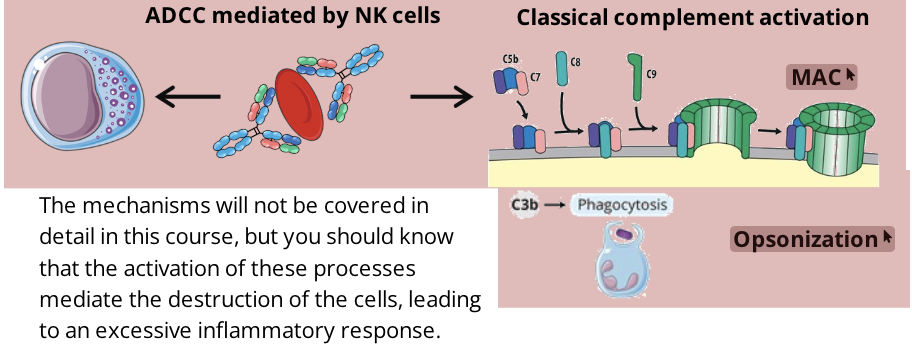
IgG, IgM, NK cells, Complement system
Mechanism of Reaction
IgGs and/or IgMs bind to antigens on the surface of cells such as erythrocytes (ie. following blood transfusion - ABO blood-group incompatibility)
once the antibodies are attached to the cell through their antigen binding region, the Fc region is free and can activate two processes called classical complement activation (leading to opsonizatoin or membrane attack complexes) and antibody dependent cell mediated cytotoxicity (ADCC)
Reaction time
reaction time of a type II hypersensitivity reaction is minutes to hours
Clinical manifestation
drug induced hemolytic anemia
some antibiotics can bind non specifically to proteins on RBC membranes and form a complex which sometimes induces complement-mediated lysis
as the RBCs rupture, the number of RBCs decreases resulting in anemia
anemia disappears when the drug is removed
penicillin is notable in inducing hemolytic anemia
transfusion reactions
depending on your blood type, you will only be able to safely receive certain blood types during a blood transfusion
this is partly due to the presence or absence of expression of a specific antigen (A or B) on your RBCs, meaning if you don’t express the antigens, you have antibodies against them
AB positive is universal recipient (no antibodies against A or B antigens), O negative is universal donor (do not have A or B antigens on RBCs)
Type III hypersensitivity (immune complex mediated)
Mediators
immune complexes (antigen-antibody complexes), neutrophils, compliment proteins
Mechanism of reaction
the reaction of antibodies with antigens generates immune complexes
when immune complexes are not cleared, they can accumulate and deposit in the tissue
these immune complexes will activate the compliment system which will indue inflammatory reactions through neutrophil attraction to the site of deposition
neutrophils release lytic enzymes as they attempt to phagocytose the immune complexes, which weakens the surrounding cell membranes ultimately causing tissue damage
Reaction time
the reaction time of a type III hypersensitivity reaction is 3-10 hours after exposure to the antigen
sometimes the reaction can take days or weeks to develop
Clinical manifestation
serum sickness
these reactions are often observed after administration of antitoxins containing foreign serum
the recipient of these antiserums (blood serum containing antibodies against specific antigens, injected to treat or protect against specific diseases) develop antibodies specific for this protein
when these antibodies circulate, they form immune complexes with the protein
after a couple of days to a week, the symptoms of serum sickness occur, including weakness, fever, and generalized vasculitis (rashes) with edema
often complexes accumulate in tissues where filtration of plasma occurs and can contribute to the pathogenesis of many other conditions such as autoimmune diseases, hepatitis, and malaria
clinical effects will subside when the antigen has been completely broken down
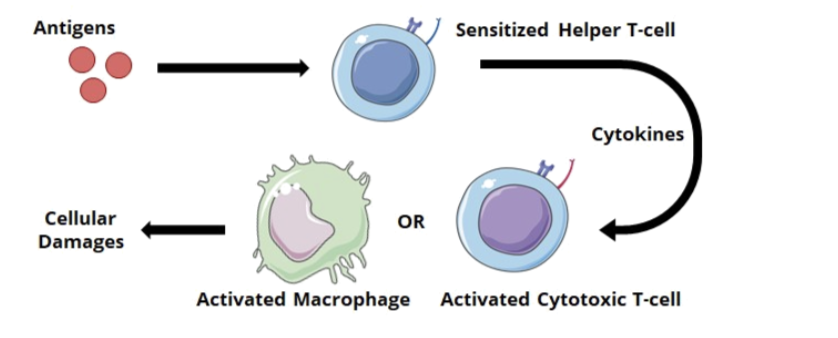
Type IV hypersensitivity (cell-mediated/delayed-type)
Mediators
CD8 cytotoxic T cells
CD4 helper T cells
Macrophages
unlike other types of hypersensitivity, type IV is not mediated by antibodies
Mechanism of reaction
after exposure to an antigen, T cells will become activated and initiate an immune response
sensitized helper T cells (specifically TH1) will release cytokines that activate macrophages or cytotoxic T cells which mediate direct cellular damage
Reaction time
the reaction of a type IV hypersensitivity has a delayed response and can take 2-3 days to develop after exposure to a particular substance
Clinical manifestation
Inflammatory Bowel Disease (IBD)
IBD is a group of conditions characterized by chronic inflammation of all or parts of the GI tract
most common are ulcerative colitis and Crohn’s disease
IBD falls into the class of autoimmune disease, where the immune system attacks the body’s own cells
Contact Dermatitis
contact dermatitis is a type of DTH response causing a red itchy rash on the skin that has been in contact with small, reactive molecules which create complexes with skin proteins
common inducers of contact dermatitis include poison ivy, formaldehyde, nickel, and cosmetics