14-thermal physics, 15-Ideal gases
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
how is thermal equilibrium achieved ?
what is thermal equilibrium ?
- to achieve thermal equilibrium between 2 objects, there will be a net flow of thermal equilibrium from the hotter object to the cooler one until they are at the same temperature.
- thermal equilibrium is achieved when there is no net transfer of thermal energy between them
what does the thermodynamic scale of temperature use as its fixed points?
how do you convert from kelvin to degrees celcius?
-the thermodynamic scale of temperature used the triple point of water, 273.16 K and absolute zero as its fixed points
-an increase of 1K ≣ 1ºC
∴ T(K) = T(ºC) + 273.16
explain the kinetic model for solids liquids gases in terms of electrostatic forces of attrcation, ± electrostatic potential energy, kinetic energy & movement
solids = closely packed together + strong electrostatic forces of attraction + molecules have negative electrostatic potential energy (negative because external energy is required to separate the molecules) + molecules have kinetic energy so can vibrate around their fixed points
liquids = molecules have a greater mean separation + still electrostatic attraction but weaker therefore, the negative electrostatic potential energy is less negative + more kinetic energy so move around more
gas = most kinetic energy so moves freely and rapidly and collides elastically with each other + collisions result in random speed and direction + electrostatic attraction is negligible therefore the electrostatic potential energy is at a maximum of 0J
what is brownian motion?
how is it caused?
-Brownian motion is the random, unpredictable movement of tiny particles when they are suspended in a fluid
-caused by molecules of fluid that are in constant motion colliding with other molecules and the tiny particles that are suspended in the fluid
What is internal energy?
what is the internal energy of a substance at absolute zero?
what type of energy is this a result of?
- internal energy is the sum of the randomly distributed kinetic and potential energies of atoms or molecules within the substance
- at absolute zero (0K), KE is zero, but, the substance still has internal energy due to its potential energy which is the electrostatic potential energy between the particles
explain the internal energies of the 3 states of matter
Which one has the most internal energy?
what has the least?
- solids have the least internal energy as KE is minimum, and electrostatic potential energy is a large negative number
- gases have the most as KE is maximum and electrostatic potential energy is negligible 0J
- liquids have more KE than solids and less electrostatic potential energy as less negative
two ways to increase the internal energy of a body; explain
- increasing the internal energy of a body can be done by increasing thermal energy which increases kinetic energy.
- during a change of state, internal energy increases with no change in temp and hence KE because the thermal energy is being used to overcome the electrostatic bonds between molecules. But internal energy increases as potential energy increases.
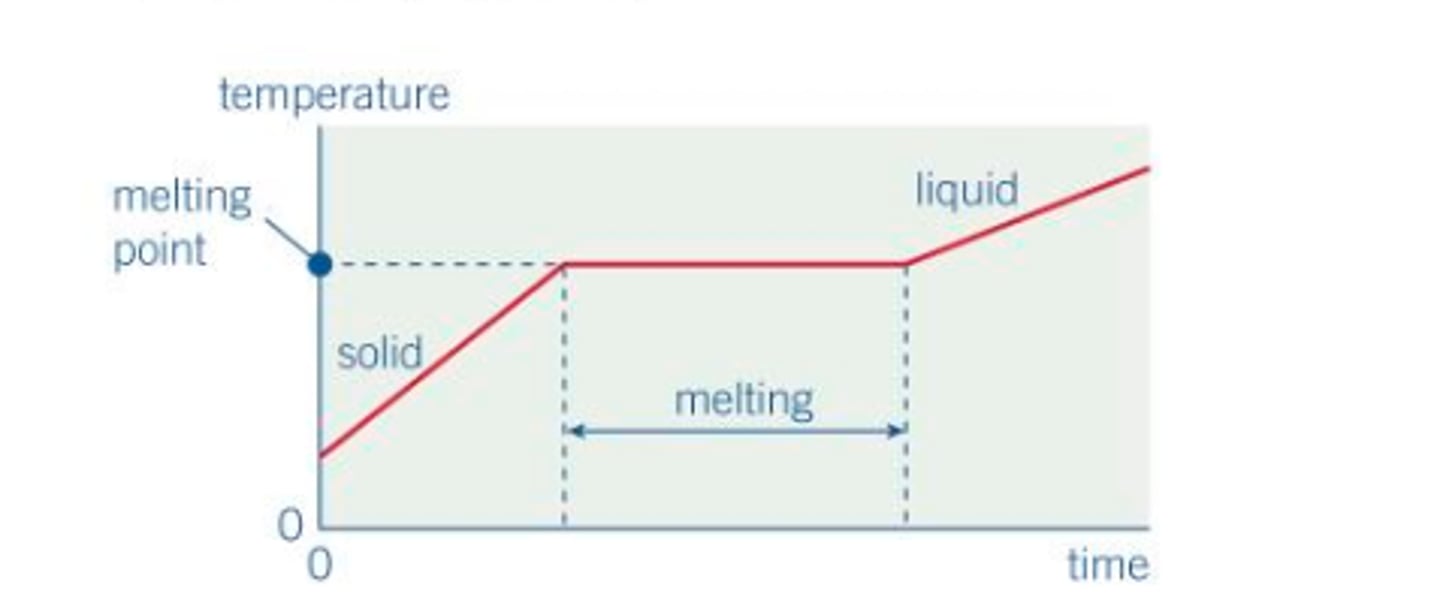
define specific heat capacity
- SHC = the energy required to per unit mass to change the temperature by 1 K
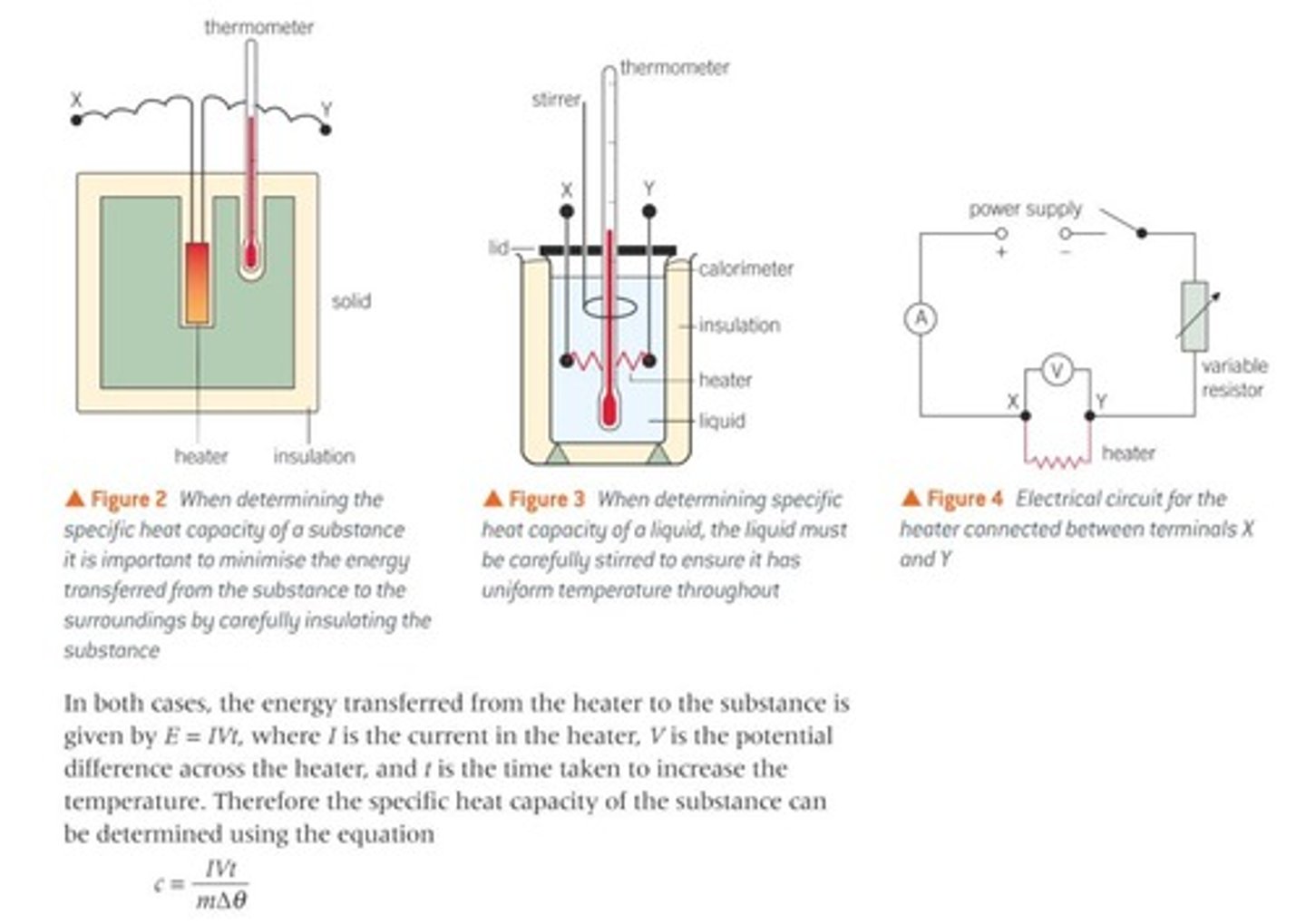
Determining specific heat capacity;
how to set up experiment?
how to carry experiment out?
what graph to plot and how to use to determine c?
setting up experiment
- heater inside solid with thermometre wrapped in insulation
- heater connected to circuit with variable resistor, ammetre, and voltmetre in parallel with resistor
- using a balance, measure the mass of the solid
carrying experiment out
- maintain a constant current and voltage across the heater by adjusting the variable resistor
- measure the change in temperature in 10 second intervals
plotting a gragh and determining the SHC
-plot a graph of Δ𝛳/Δt
E = mcΔ𝛳 E/t = mc(Δ𝛳/t) E/t = Power
P=mc(Δ𝛳/t) where, m = mass, P = IVt, Δ𝛳/t = gradient, c = SHC
rearranging for c,
c = P/m x gradient (Δ𝛳/t)
define specific latent heat?
what is specific latent heat of fusion?
what is specific latent heat of vaporisation?
-Specific Latent Heat is defined as the energy required to change the phase per unit mass while at constant temperature
-Latent heat of fusion = solid ⇔ liquid
-Latent heat of vaporisation = liquid ⇔ gas
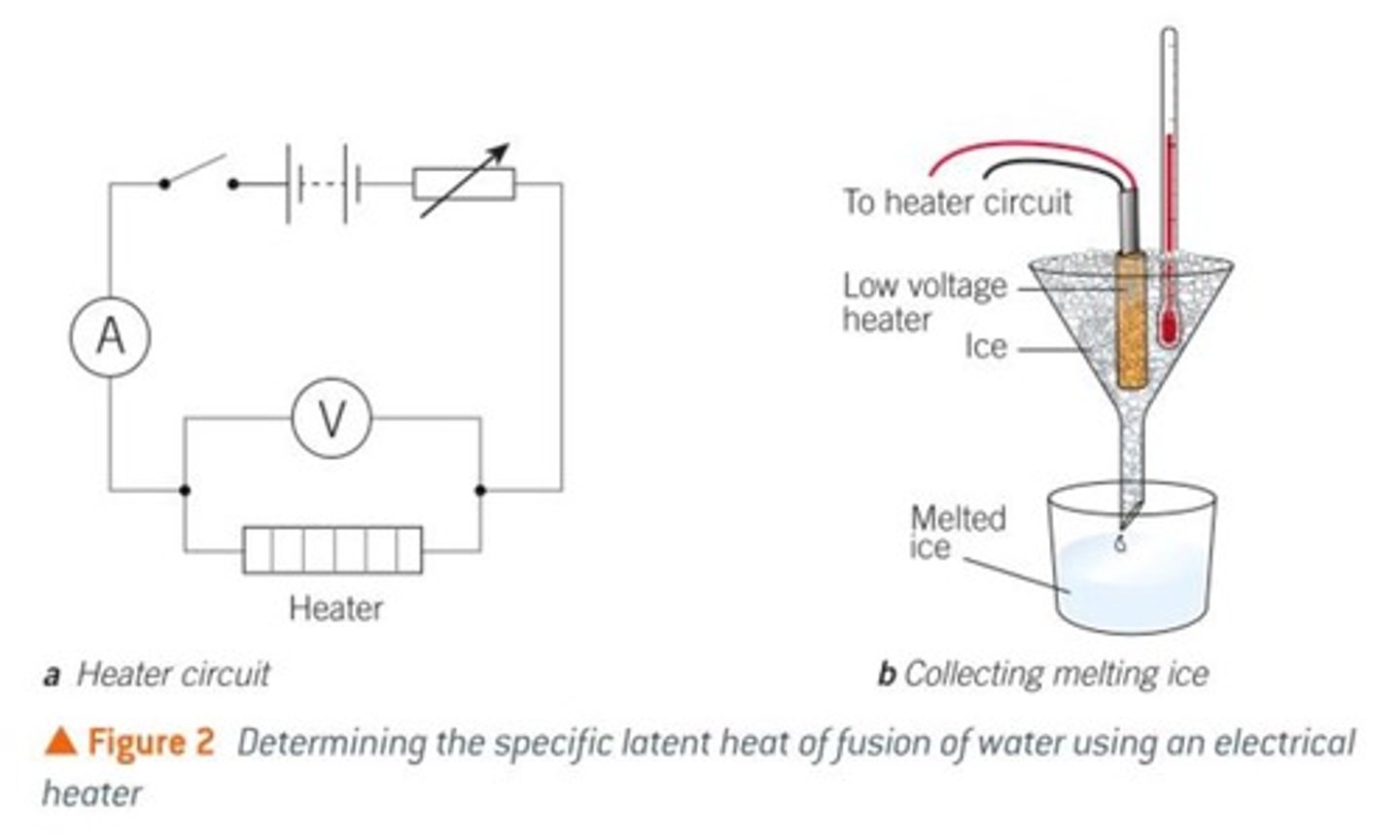
determining specific latent heat of fusion,
How to setup the experiment?
how to carry out?
how to calculate specific latent heat of fusion?
setting up the experiment
- heater connected to voltmetre in parallel, ammetre in series, variable resisitor in series to maintain constant current and voltage
- heater inside ice with themometre to ensure no change in temp
- ice must be at melting point 0ºC
- balance to measure the mass of liquid at end
carrying out the experiment
- using a timer, time how long it takes for a large amount to melt
- measure mass of melted ice
determing specific latent heat of fusion
- using IVt/m, L can be determined
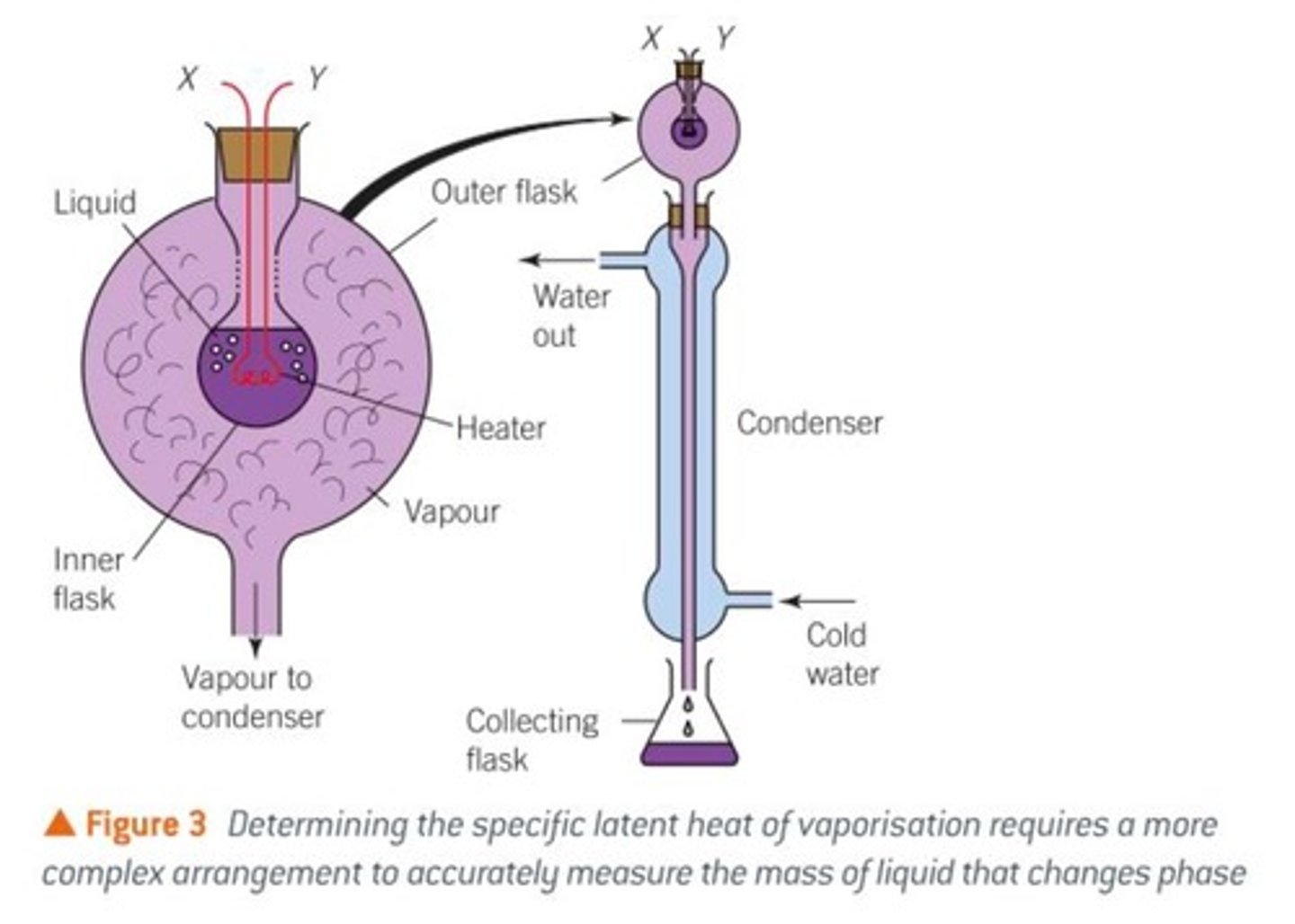
determining latent heat of vaporisation
setting up the experiment
- inner flask contains liquid and heater connected to circuit (volemeter, ammerter, variable resistor)
- inner flask inside outer flask containing vapour
- outer flask connected to a condensor with a collecting flask at the bottom
carrying out the experiment
- time how long it takes to vaporise liquid
- measure the mass of the vaporised liquid that condensed again
calculating specific latent heat of vaporisation
- L = IVt/m
combining specific heat capacity and specific latent heat:
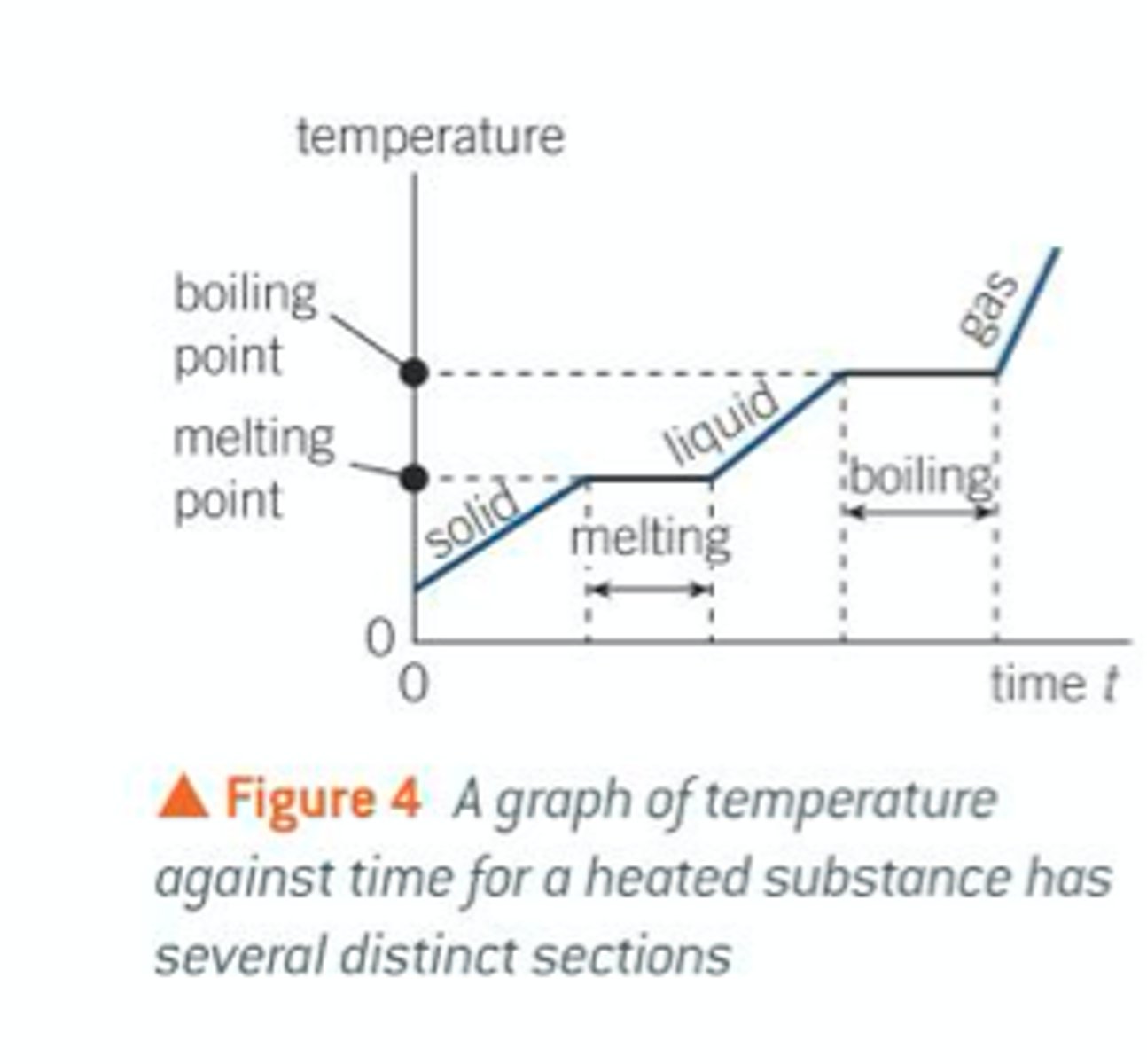
how to calculate the total energy required to change a substance from solid to gas ?
what does the graph look like?
to calculate the total energy to change a substance form solid to gas;
E = mc(solid)Δ𝛳
+ E = mL(fusion)
+ E = mc(liquid)Δ𝛳
+ E = mL(vaporisation)
what is avogadros constant?
what is the formula used to find number of atoms or molecules in a substance?
what does each term represent?
-avogadros constant = 6.02x10^23
- the total number of atoms or molecules in a substance is given by ; N = n x Na where N is total number, n is number of moles, Na is avogadros constant
what does molar mass measure?
what is each individual atoms molar mass?
what is the formula to find the total mass from the molar mass of a substance?
-molar mass is the mass of one mole
-molar mass is the same as the nucleon number of atoms
-to find the total mass from molar mass, we multiply the molar mass by the number of moles we have to find the total mass;
m = n x M
m = total mass, n = number of moles, M = molar mass
what are the 5 assumptions of an ideal gas?
- gas contains large num of atoms or molecules moving in random directions with random speeds
- the atoms or molecules of the gas occupy a negligible volume compared with the total volume of the gas
- the collisions of the atoms with each other & the container walls are perfectly elastic
- the time of collision between atoms or molecules is negligible compared to the time between collisions
- electrostatic forces between atoms or molecules are negligible except during collisions
What is Boyle's law ?
what is its equation for the constant?
Boyle's law states that if the temperature and mass of a gas remain constant, then the pressure of an ideal gas is inversely proportional to its volume
P ∝ 1/V
pV = constant
What is Charles' Law?
what is the equation for the constant?
Charles' law states that for a fixed mass of a gas at a constant temperature, volume is directly proportional to temperature
V ∝ T
V/T = constant
What is the Pressure Law?
For a fixed mass of a gas at constant volume, the pressure is directly proportional to the absolute temperature
statement: For a fixed mass of gas at constant volume, the pressure P is directly proportional to the temperature
p ∝ t
p/t = constant
after combining Boyle's law, Charles' law, and Gay-lussac's law, you get the Combined Gas Law
what is the equation?
combining all three laws, you get the Combined Gas Law equation;
PV/T = k
what is the value of the molar gas constant R?
what does it represent?
what does the Combined Gas Law equation become?
what is the new name?
For one mole of an ideal gas, the constant in the combined relationship is called the molar gas constant R = 8.31 J K^-1 mol^-1
so for n moles of gas,
PV/T = nR
this is called the equation of state of an ideal gas
Investigating Boyle's law
Boyle's law states that the pressure exerted by a fixed amount of gas is inversely proportional to its volume at a constant temperature. To investigate this experimentally, a sealed syringe can be filled with gas and connected to a pressure gauge. The syringe can be used to vary the volume of the container, and the values for volume and pressure recorded. When a graph of pressure against 1/volume is plotted, a straight line should be produced, showing a constant relationship. To increase accuracy in this experiment, the syringe should be lowered slowly so that no heat is produced from friction.
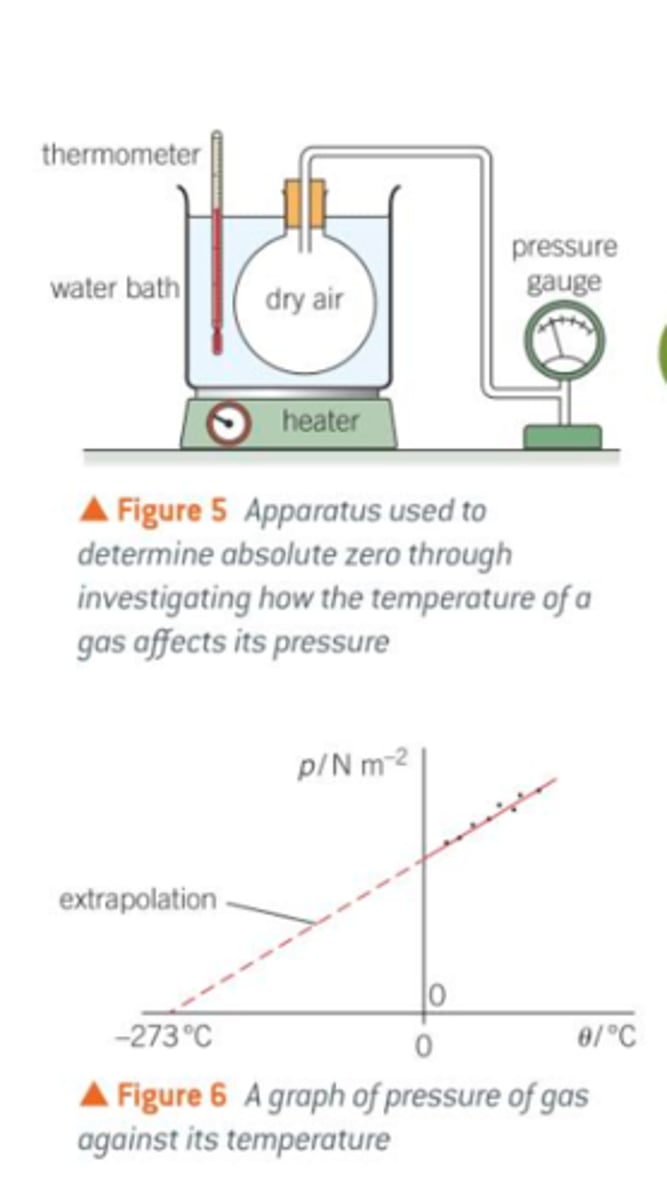
Investigating the Pressure Law
- immerse a stoppered flask of air in a water bath with a digital thermometer
- connect the stopper to a bourdon gauge using a tube
- record the temp of water and pressure in flask
- Increase the temperature of the water bath, then stop and stir the water to allow the temperature to be uniformly distributed and allow some time for the heat to be transferred to the flask of aire
- record new temp and pressure
- increase temp of water bath again and repeat to get many readings
- Repeat the whole experiment
- plot pressure against temp and draw line of best fit
- line should be a straight line showing P ∝ T
- can estimate absolute zero by extrapolating
what is the r.m.s. speed representing?
how to get the r.m.s. speed?
-the r.m.s. speed is a measure of the average speed of particles in a gas .
-the root mean square speed, c, is given by summing the square of all individual velocities of the molecules and then dividing by the number of molecules, N, and then finding the square root of this value.
What is the formula linking pressure, volume, number of particles, and r.m.s. and mass?
-the r.m.s. speed can be used to find the pressure of a gas at the microscopic level using pV= ⅓Nm |c^2|
p = pressure
V = volume
N = number of molecules
m = mass
c^2 BAR= m.s. speed