Elements , compounds and mixtures
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
Element
pure substance that consists entirely of one type of atom
Compound
A substance made up of atoms of two or more different elements joined by chemical bonds
Mixture
A combination of two or more substances that are not chemically combined
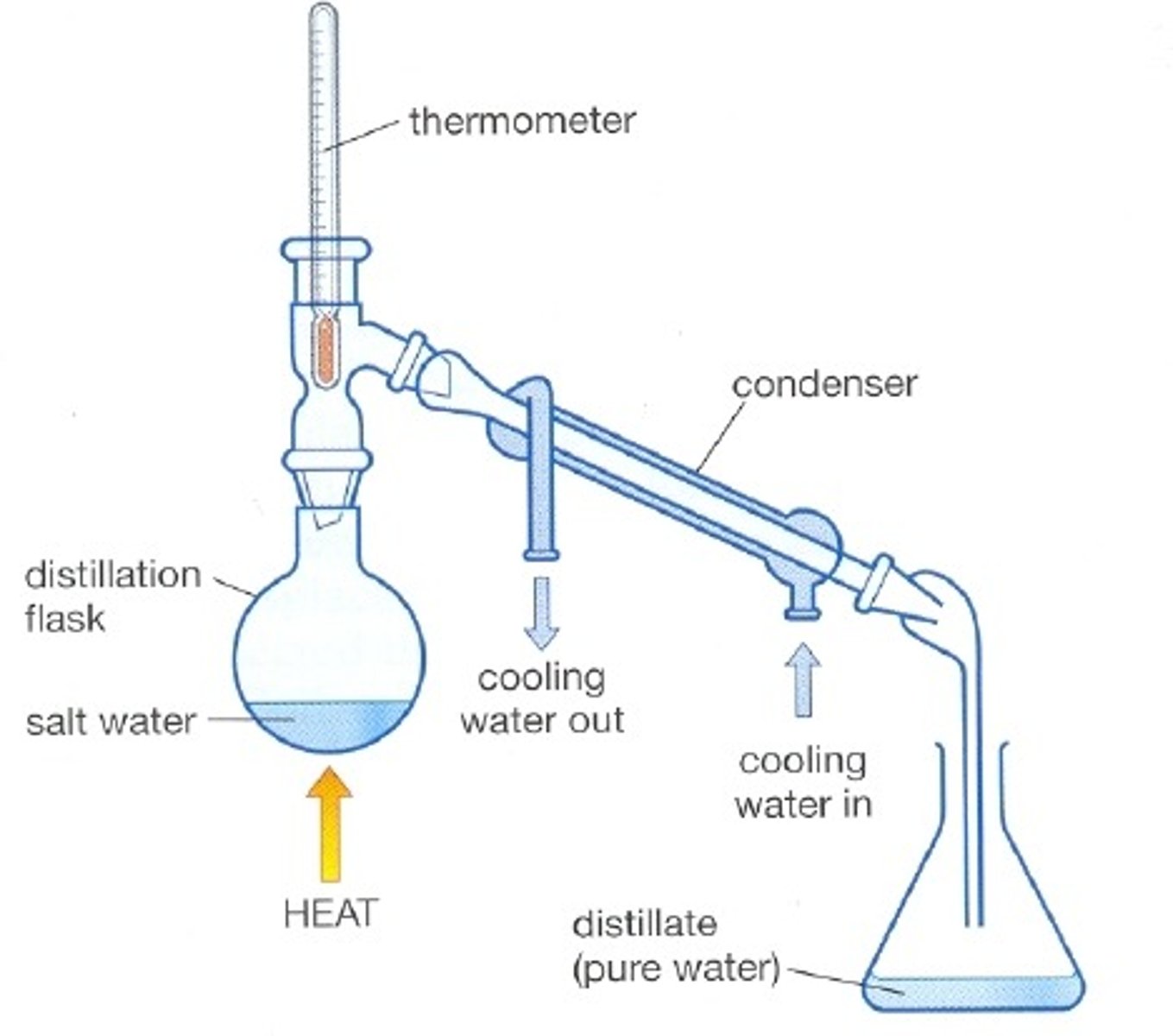
Simple distillation
Used to separate a liquid from a solution
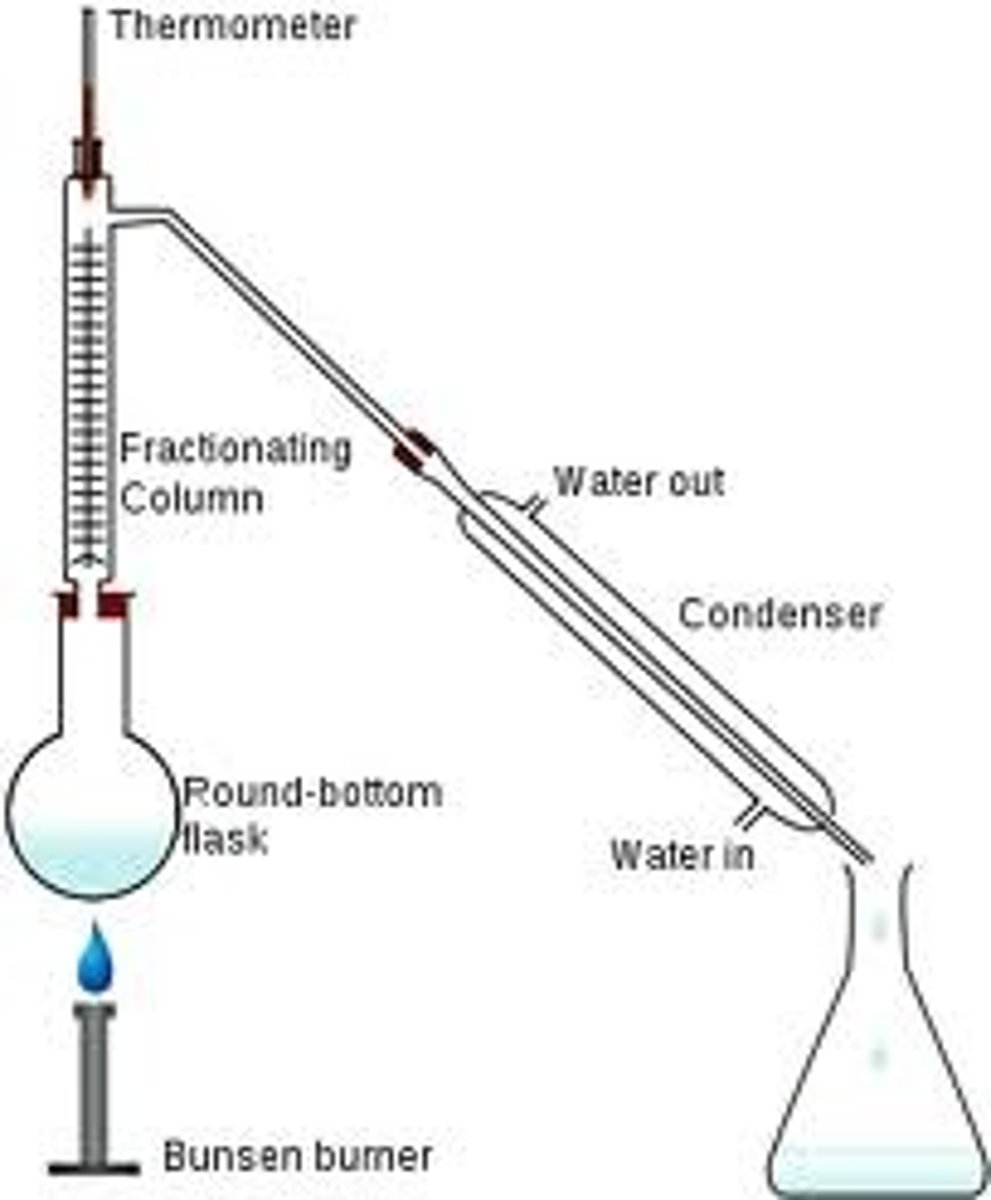
Fractional distillation
separation of a liquid mixture into fractions differing in boiling point (and hence chemical composition) by means of distillation, typically using a fractionating column.
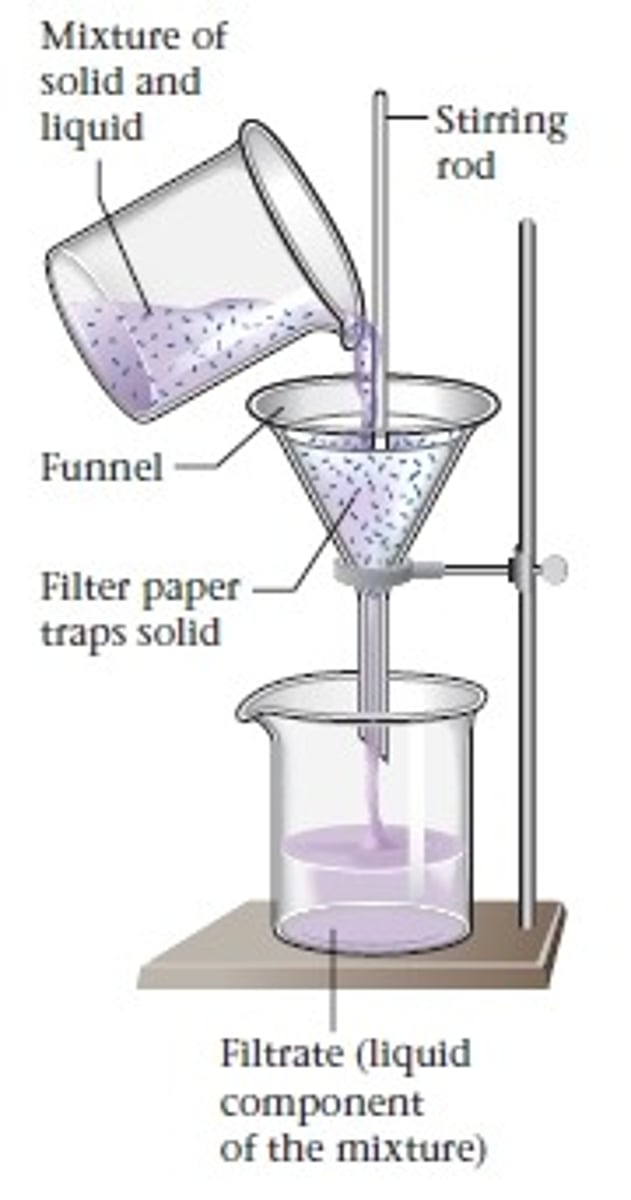
Filtration
the process that separates a solid from the liquid in a mixture
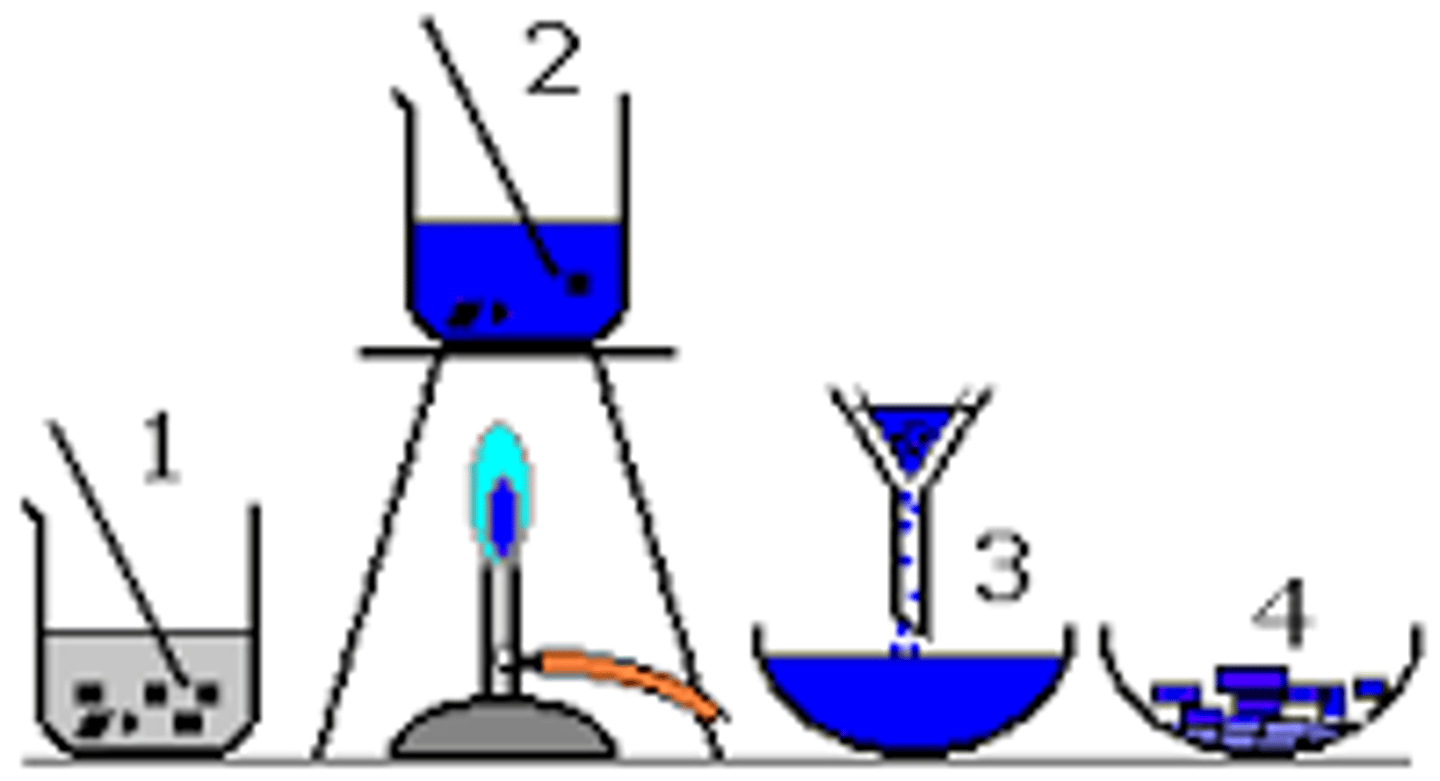
Crystallisation
Formation of crystals as a dissolved substance solidifies
Chromatography
A technique that is used to separate soluble solids mixed together, like colours
Atom
Smallest particle of an element
Molecule
A group of atoms bonded together
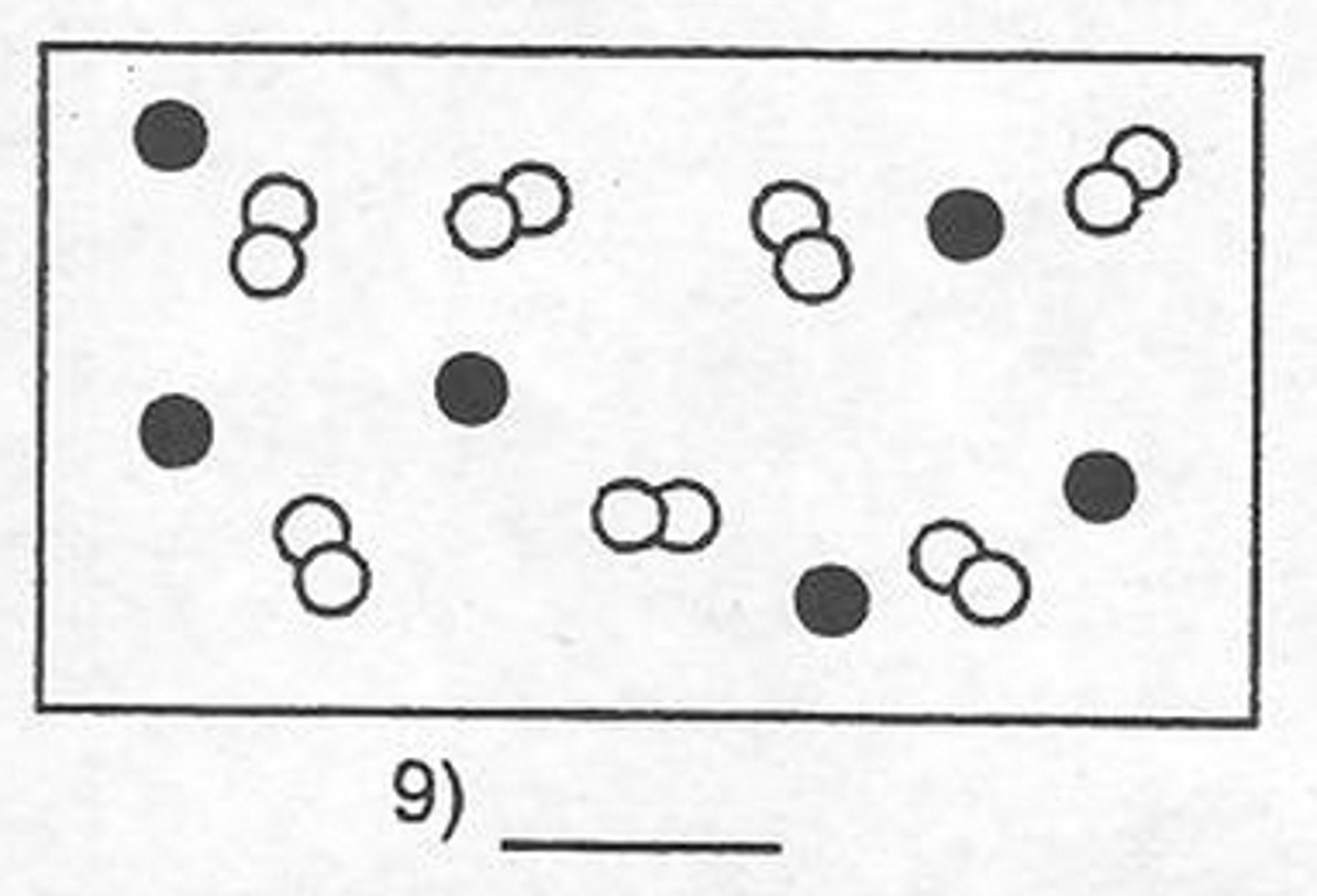
Mixture of elements
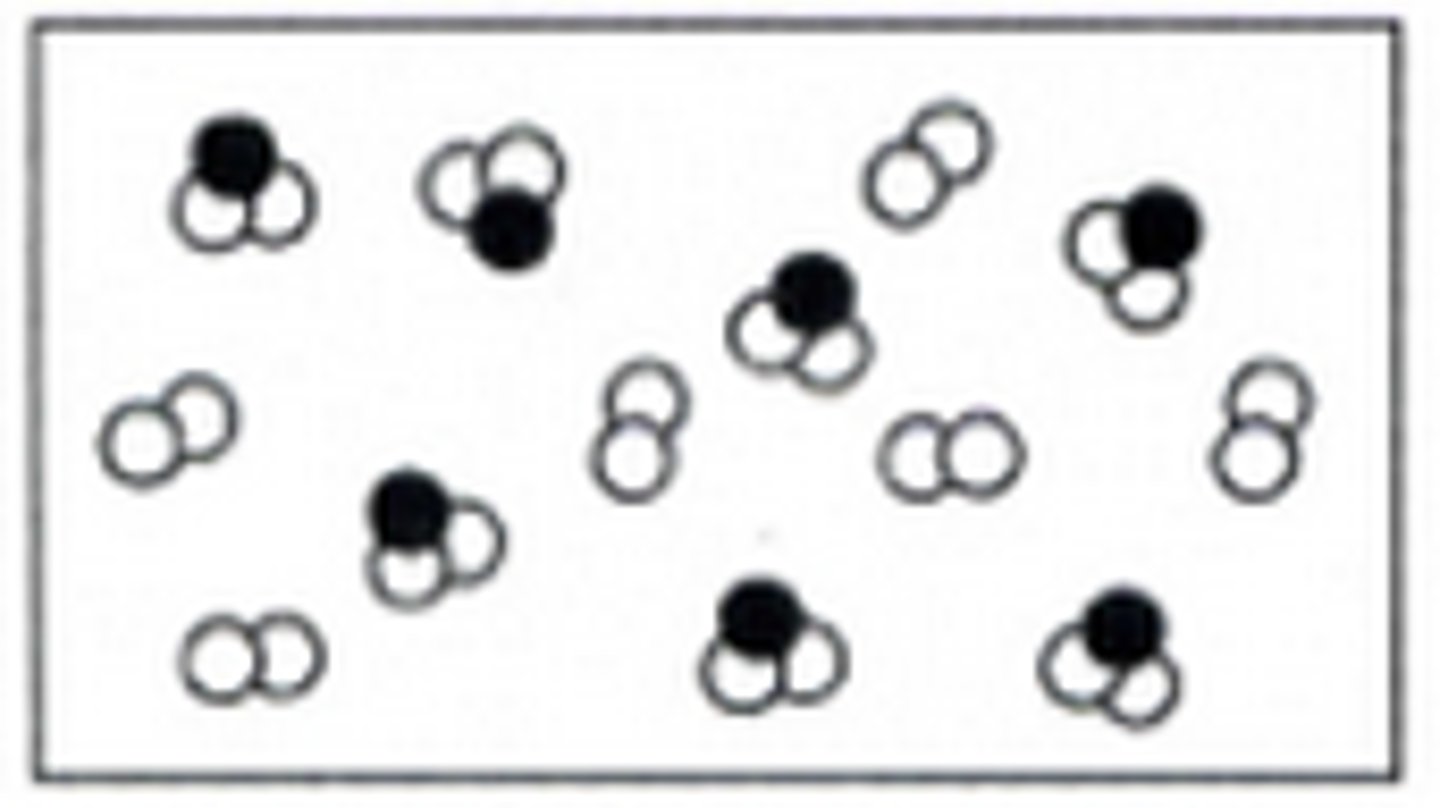
Mixture of element and compound
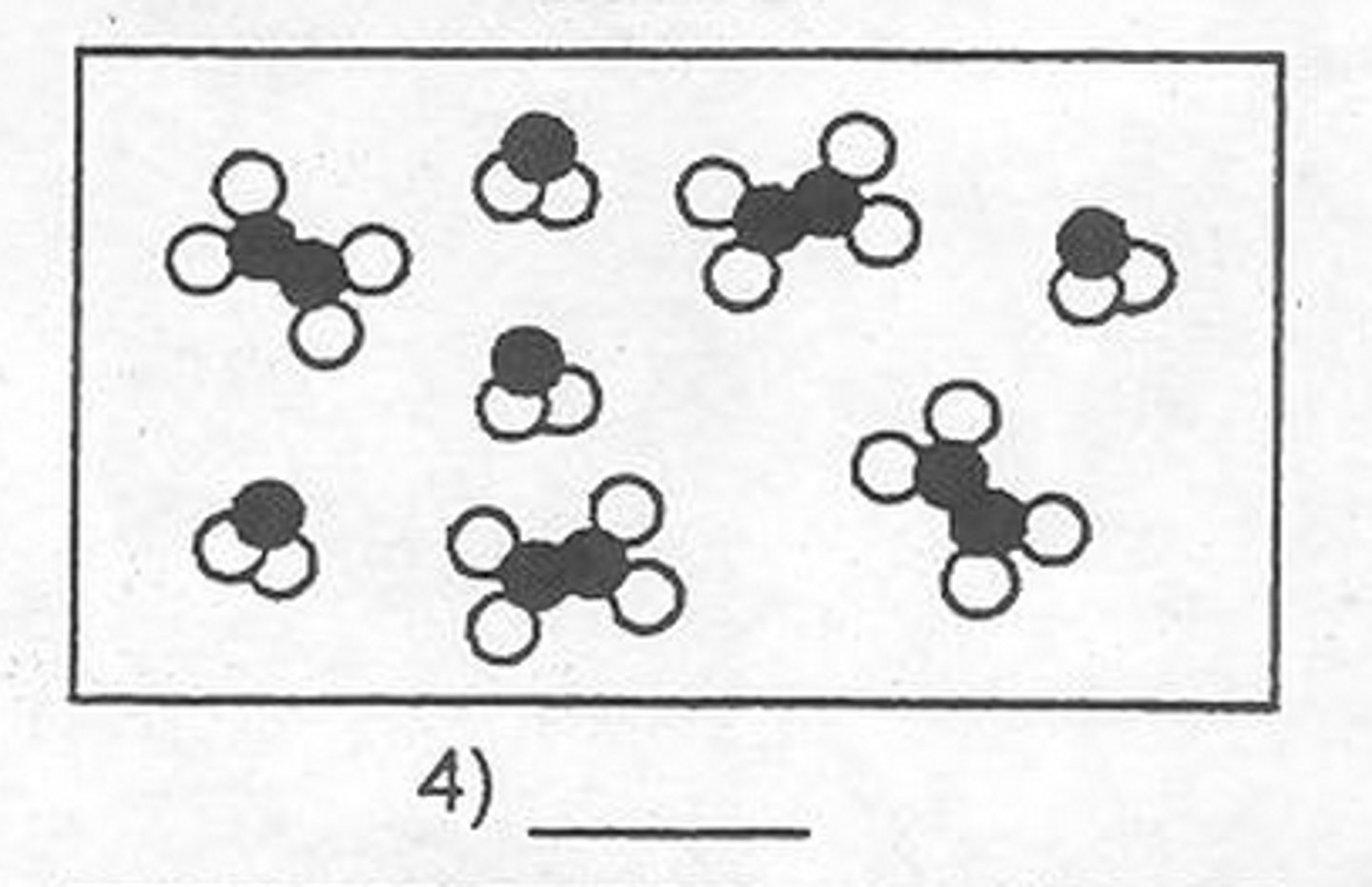
Mixture of compounds
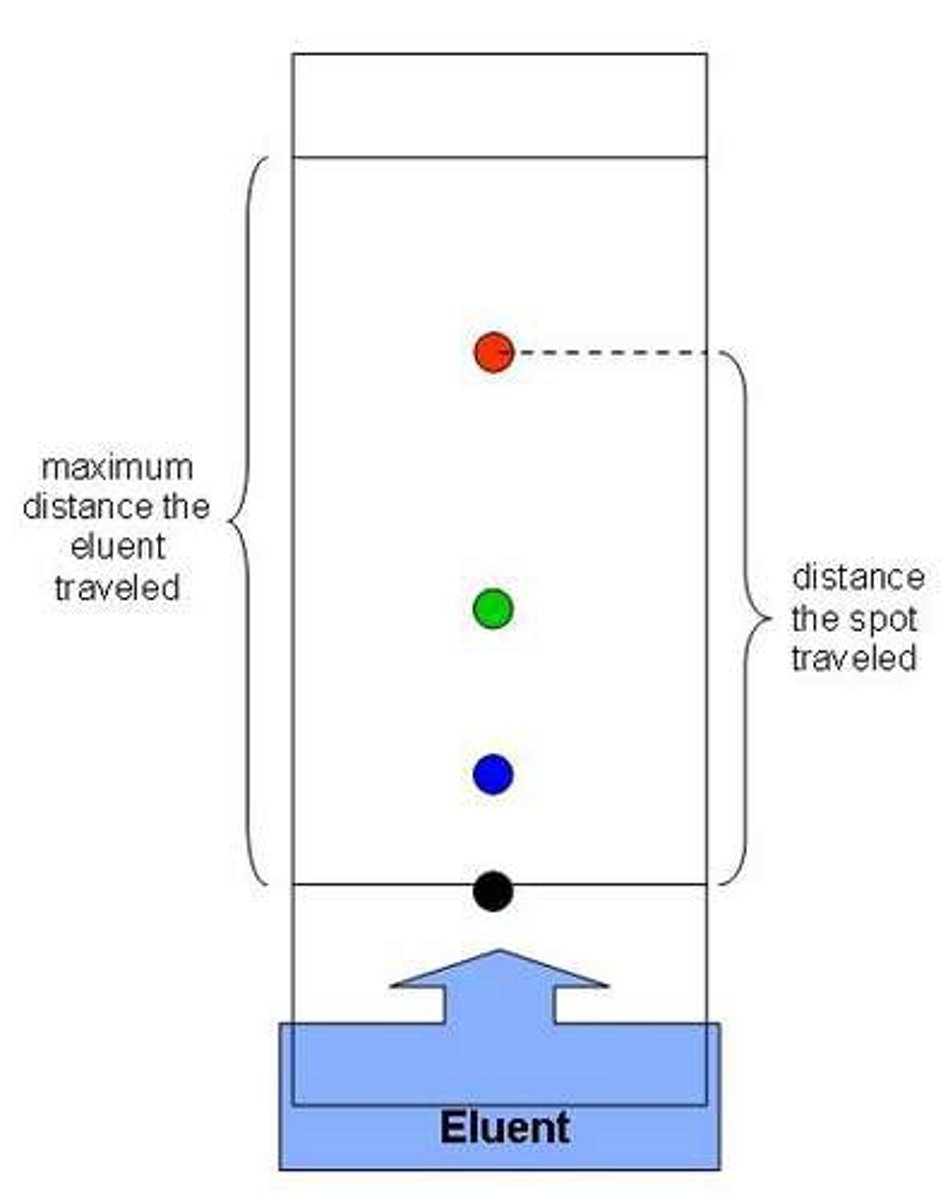
Rf value
Distance travelled by substance / distance travelled by solvent