W4L3: Reactions of alkanes
0.0(0)
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
1
New cards
Reactions and uses of alkanes
Alkanes are chemically inert – they do not react readily
Free radical oxidation
Cracking
Free radical substitution
2
New cards
Free radical oxidation
They are burnt to produce carbon dioxide and water
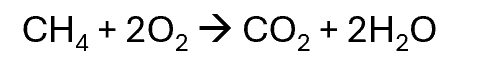
3
New cards
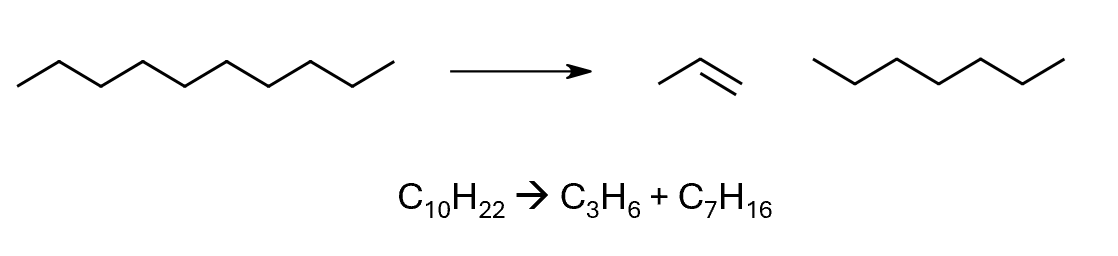
Cracking
large hydrocarbon molecules can be “cracked” into smaller, more useful molecules (e.g. petrol). High temperature, high pressure and special catalysts needed (atypical reaction conditions).
4
New cards
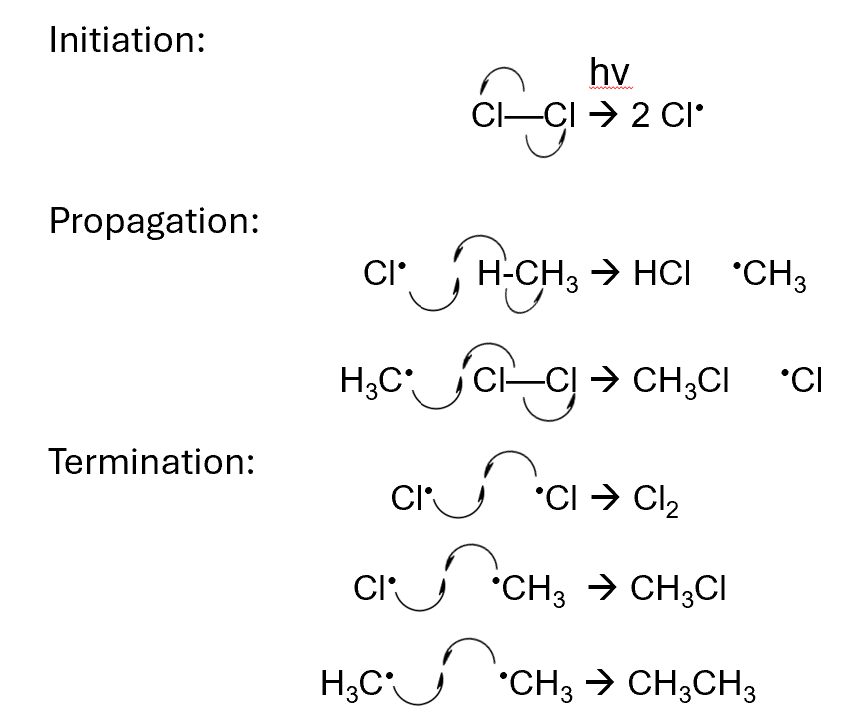
Free radical substitution