metabolic diversity
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
electron donors
chemoorganotrophy: organic molecules
chemolithotrophy: inorganic molecules
phototrophy: uses light energy to reduce compounds and then use these as electron donors
electron acceptors
respiration: inorganic /organic molecules
fermentation: organic molecules
gibbs free energy change

electron transport
electron transfer never occurs randomly: moves from a low reduction to a high reduction potential
genrates proton motive force
metabolism is underpinned by the production of 2 sources of energy: reducing energy (NADH, NADPH and FADH2) and ATP
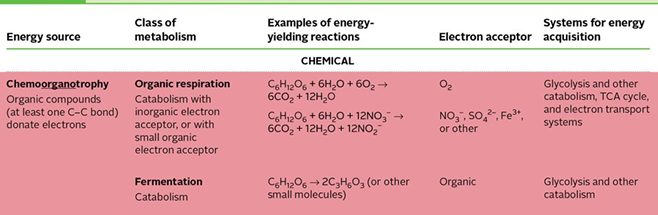
chemoorganotrophy
a wide range of organic compounds can be used as a source of electrons: carbs, lipids, peptides, aromatic compounds
produces acetyl-coA and pyruvate (metabolites)
what makes metabolism complicated is that there is more than 1 way to metabolise glucose (glycolysis, entner-doudoroff and pentose phosphate pathway)
elevtron transport (inorganic) occurs via:
cytochromes, quinones and iron-sulfur proteins
anyoxygenic respiration is important to exploit a wide range of ecological niches
aquatic water quality and denitrification
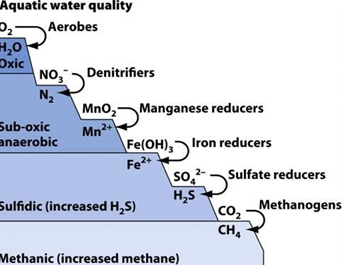
methanogenesis
acetate: CH3COO- + H+ + CH4 + CO2
methanol: 4CH3OH + 3CH4 + CO2 +2H2O
what differentiates anaerobic respiration and fermentation
anaerobic respiration:
uses inorganic molecules (other than O2) or organic molecules as terminal electron acceptors via a membrane bound respiratory chain
ATP produced by oxidative phosphorylation via the PMF
fermentation:
use of organic molecules as electron acceptors, without use of a respiratory chain
ATP produced by substrate-level phosphorylation in the cytoplasm
fermentation energy yields are low; cells grow more slowly than when they respire
chemolithotrophy: important properties
chemolithotrphs use CO2 as a carbon source to produce organic molecules via calvin cycle, reverse TCA cycle
they can also use more complex molecules (acetate)
to fix carbon, they require NADH; this requires consumption of H+ for a reverse electron flow process
chemolithotrophy: electron acceptors
O2: H2 + ½ O2 → H2O
SO42-: 4H2 + SO42- + H+ → HS + 4H2O
CO2: 4H2 + CO2 → CH4 + 2H2O
iron oxidation
Reduced iron Fe2+ can be oxidised to Fe3+ at low pH 2Fe2+ + ½ O2 + 2H+ → 2Fe3+ + H2O
Ferric ions (Fe3+) form insoluble ferric hydroxide [Fe(OH)3] as the pH Is getting lower Fe3+ + 3H2O → Fe (OH)3 + 3H+
nitrogen oxidation
Ammonia and nitrites can be used as electron donors to produce nitrates (NO3-)
Nitrification (NH4+ → NH2OH → NO2- → NO3-) occurs in aerobic conditions, carried out by Nitrosomonas and nitrosobacter
Anammox (NH4+ + NO2- → N2 + 2H2O) occurs in anaerobic conditions, carried out by planctomycetes
sulfur oxidation
Several sulfur derivatives can be used as electron donors to produce sulfuric acid (H2SO4)
H2S → SO → S2O32- → H2SO4
Use of acid-producing microbes in biomining
phototrophy
oxygenic photosynthesis and anoxygenic photosynthesis
bacteriorhodospin
a very abundant light-driven proton pump in archeal membranes.
Contains a pigment (retinal) that undergoes conformational change once excited by light (trans → cis).
this triggers the transfer of a proton to Asp85. Deprotonated retinal pushes against helix F, opening a channel on the cytoplasmic side; this induces deprotonation of retinal from Asp96
Asp96 undergoes reprotonation. Asp96 transfers a proton outside through hydrogen bonding via water molecules and other residues
oxygenic photosynthesis (cyanobacteria)
no chloroplast
their photosynthetic apparatus is variable; most often made of thylakoids
light is captured by light harvesting complexes (contains several pigments which can use light energy at various wavelengths) which channels energy to a reaction centre
the oxygenic z pathway
2 distinct photosystems are excited by light
light provides energy to strip the electron from water yielding H+
the electron flow is used to pump protons outside the cell and reduce NADP+
the H+ gradient is used to generate ATP
NADPH and ATP are used to fix CO2 and make glucose
green sulfur bacteria
light is captured by antenna complexes in organelles called chlorosomes
photon energy is transferred to the PSI reaction centre
anoxygenic photosynthesis: PSI donates an electron to the ETC, electron transport pumps protons outside the cell and reduces NADP+ via ferredoxin. The proton gradient is used to generate ATP. PSI recieves electrons from inorganic sulfur derivatives
purple bacteria
light is captured by antenna complexes in organelles called chromatophores
photon energy is transferred to the PSII reaction centre
PSII donates an electron to a cyclic ETC
electron transport pumps protons outside the cell, the H+ gradient is used to generate ATP (cyclic photophosphorylation)
NADH is produced by reverse electron flow; electrons are transferred from reduced ETC components (with a more positive reducing potential). This reaction is not thermodynamically favourable and consumes energy
electrons transferred to NAD+ by ETC components are replenished by inorganic or organic compounds