Stoichiometry & General Concepts
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
What is a mole?
It is an SI unit used to measure the amount of any substance.
What mole is equal to? Avogadro’s number
6.022 × 1023
What is molar mass?
The sum of the mass of all atoms found in one mole of a substance. It is expressed in units of grams per mole. Molar mass is = to the atomic mass
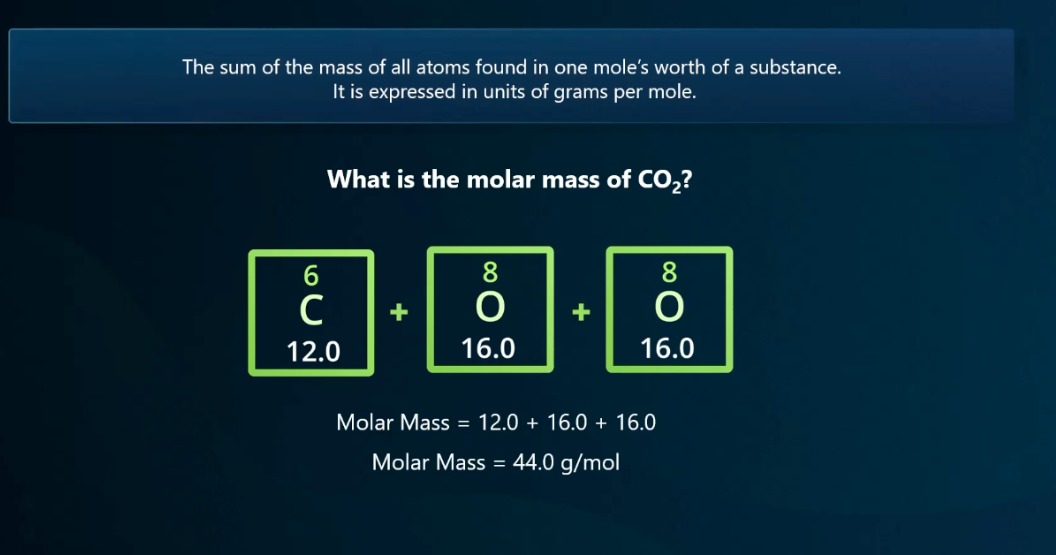
What is the equation to calculate moles? (Mass to Moles)

How many atoms of O are in 1.5 moles of O2

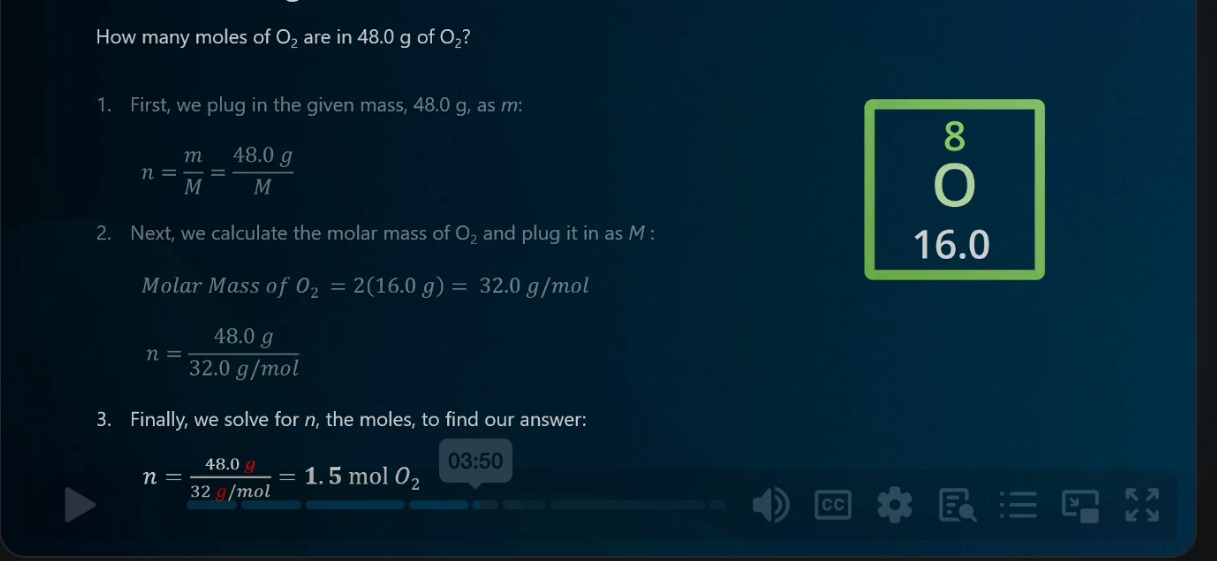
How many moles of O2 are in 48.0 g of O2?
What is mole ratio? (Mole-to mole ratio)
It is a ratio between the amount of moles of any two molecules involved in a chemical reaction
What can be used to predict how much product a reaction forms?
Mole ratio
How many moles of carbon are present in 1 mol of C2H6?
two carbons times 1 mole
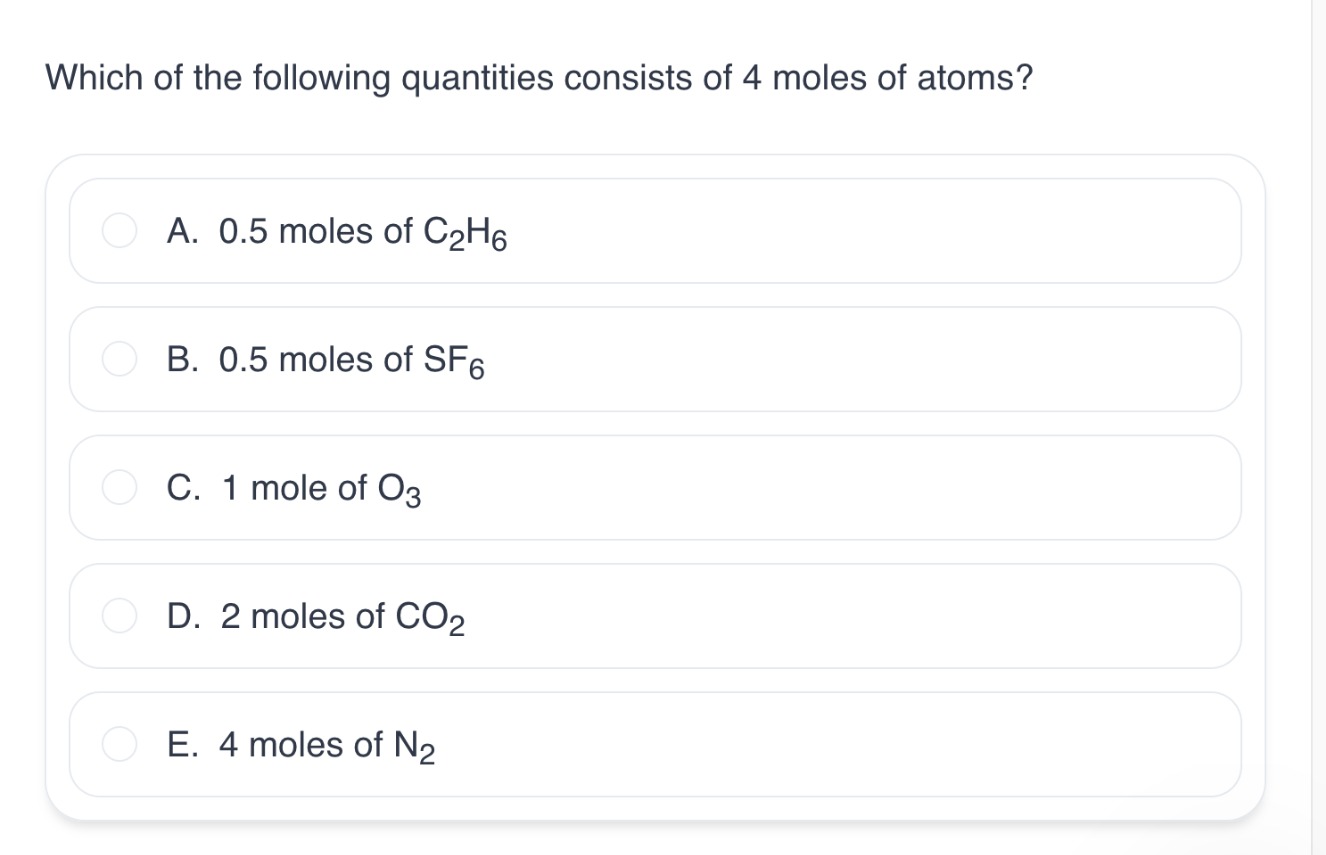
Which of the following quantities consists of 4 moles of atoms?
Count the amount of atoms per molecule. Out of all of them, option A has 8 atoms per molecule. This implies that 0.5 moles of that molecule contain 4 moles of atoms → 0.5 X 8 =4

What is percent composition?
Is the percent of the compound’s mass that is made up by that element
What is the percent composition formula?
look at picture
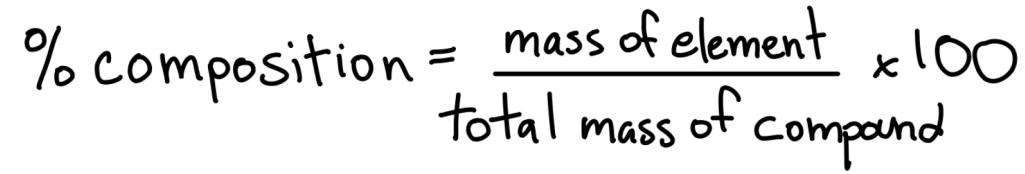
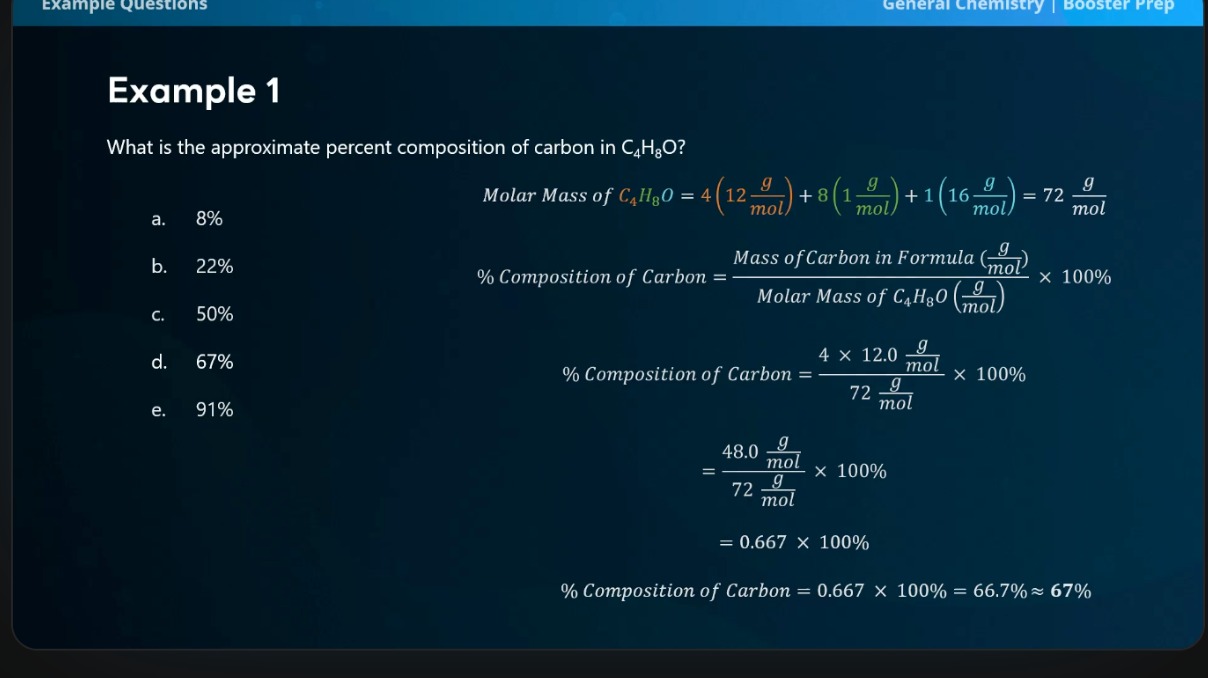
What is the approximate percent composition of carbon in C4H8O?
look at picture
A compound has the following percentage composition: 24% carbon, 28% hydrogen, 48% oxygen. In 100 grams of the compound described, what is the mole ratio between oxygen and carbon?
first find the moles of oxygen → 48g x (1 mol/ 16/g)=3
moles of carbon→ 24g x (1 mol/ 12/g)=2
Ratio would be 3:2

The regular formula is called?
Molecular formula
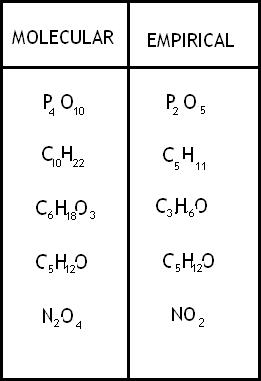
The reduced formula is known as?
Empirical formula
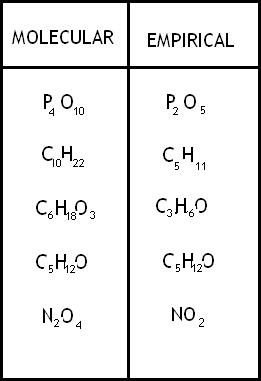
What is the molecular formula of the compound HCO3, given that the molar mass is 90.0g/mol?
First, we calculate the molar mass of the given empirical formula. 1g.mol + 12g/mol+ 2(16g/mol)= 45 g/mol
Next, we divide the given molecular molar mass by the molar mass calculated for the empirical formula: 90 g/mol ÷ 45 g/mol = 2
Finally, we multiply each subscript in HCO2 by 2. H2C2O4
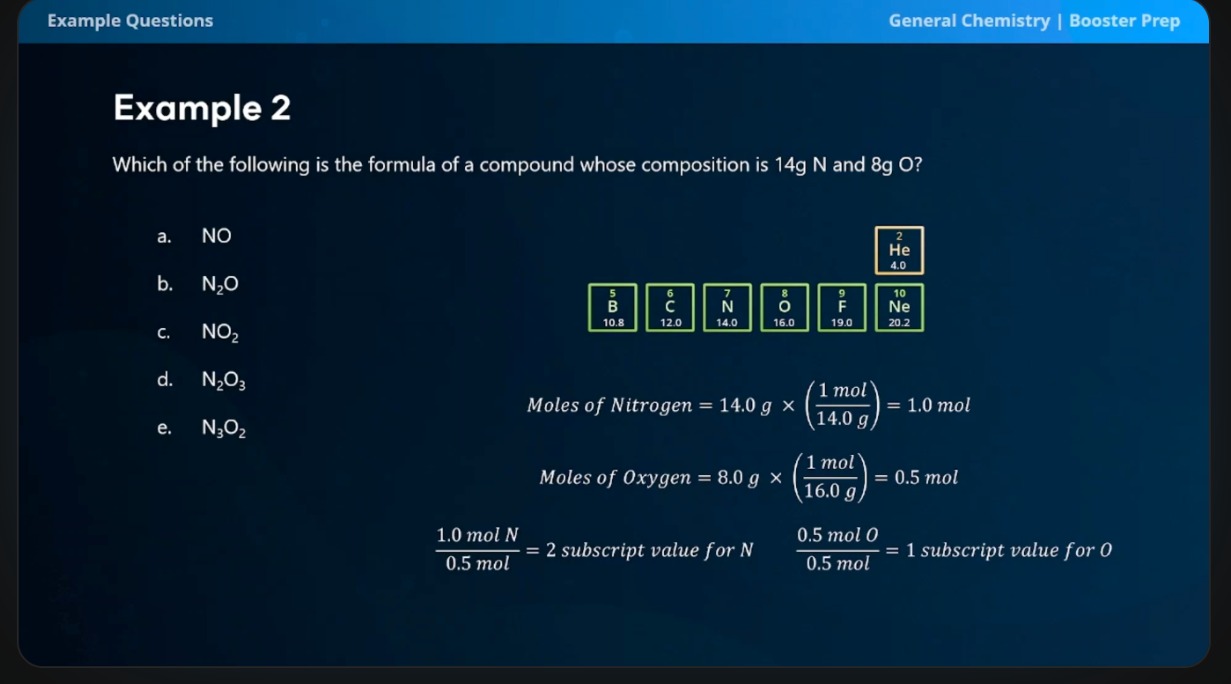
Which of the following is the formula of a compound whose composition is 14g N and 8g O?
look at piccture
What is the empirical formula of a compound that is 56.0% iron (M.M of 56 g/mol) and 40,0% oxygen (M.M of 16 g/mol)
First, we convert the percentages to grams, assuming a 100g sample, resulting in 56g of iron and 40g of oxygen.
Next, we find the number of moles: 56g Fe ÷ 56 g/mol = 1 mol Fe and 40g O ÷ 16 g/mol = 2.5 mol O.
Finally, we divide by the smallest number of moles (1) to get the empirical formula: FeO2.5 or Fe2O5 after adjusting for whole numbers.
What is a coefficient?
The number of each molecule that is used to balance the chemical equation (can be changed)
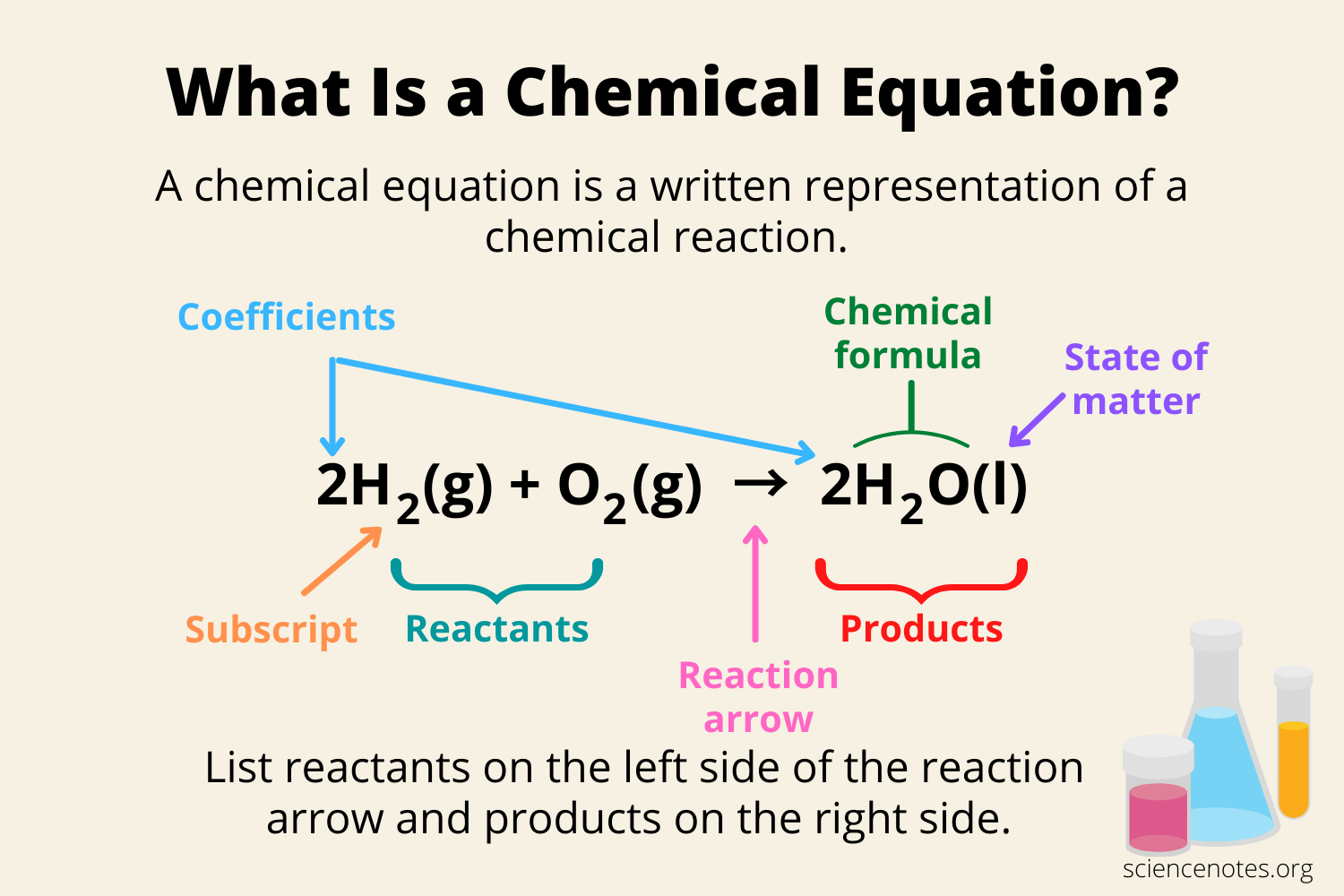
What is a subscript?
A number in a chemical formula that indicates the number of atoms of an element in a molecule. (can not be changed)
How many moles of ammonia can be expected from the reaction of 250 moles of N2 gas?
Look at picture


Based on the chemical reaction, how many moles of oxygen are needed for the complete combustion of 2 moles of methane?
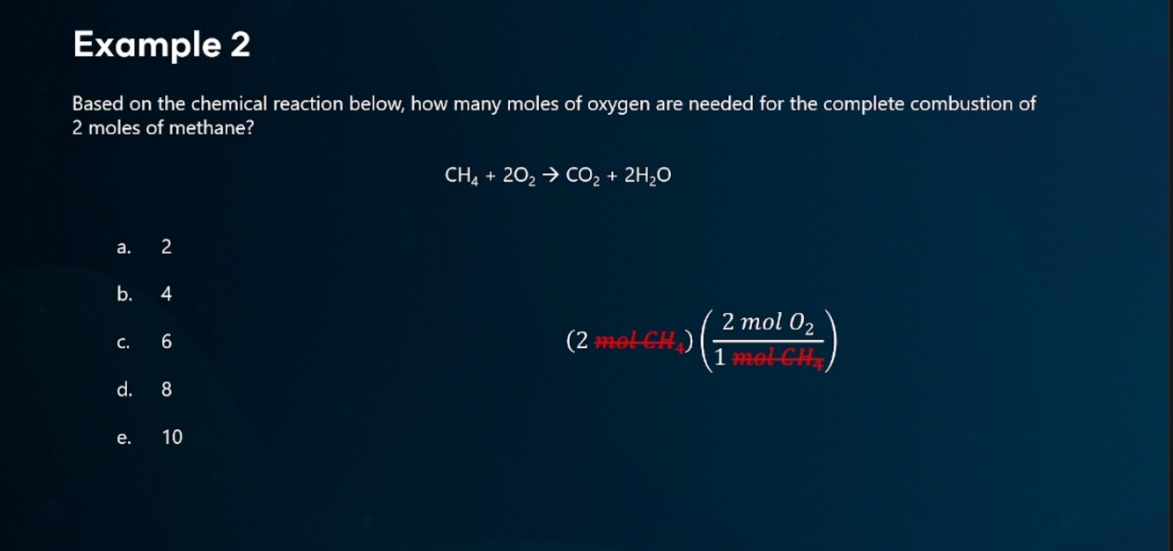

slove problem


solve problem
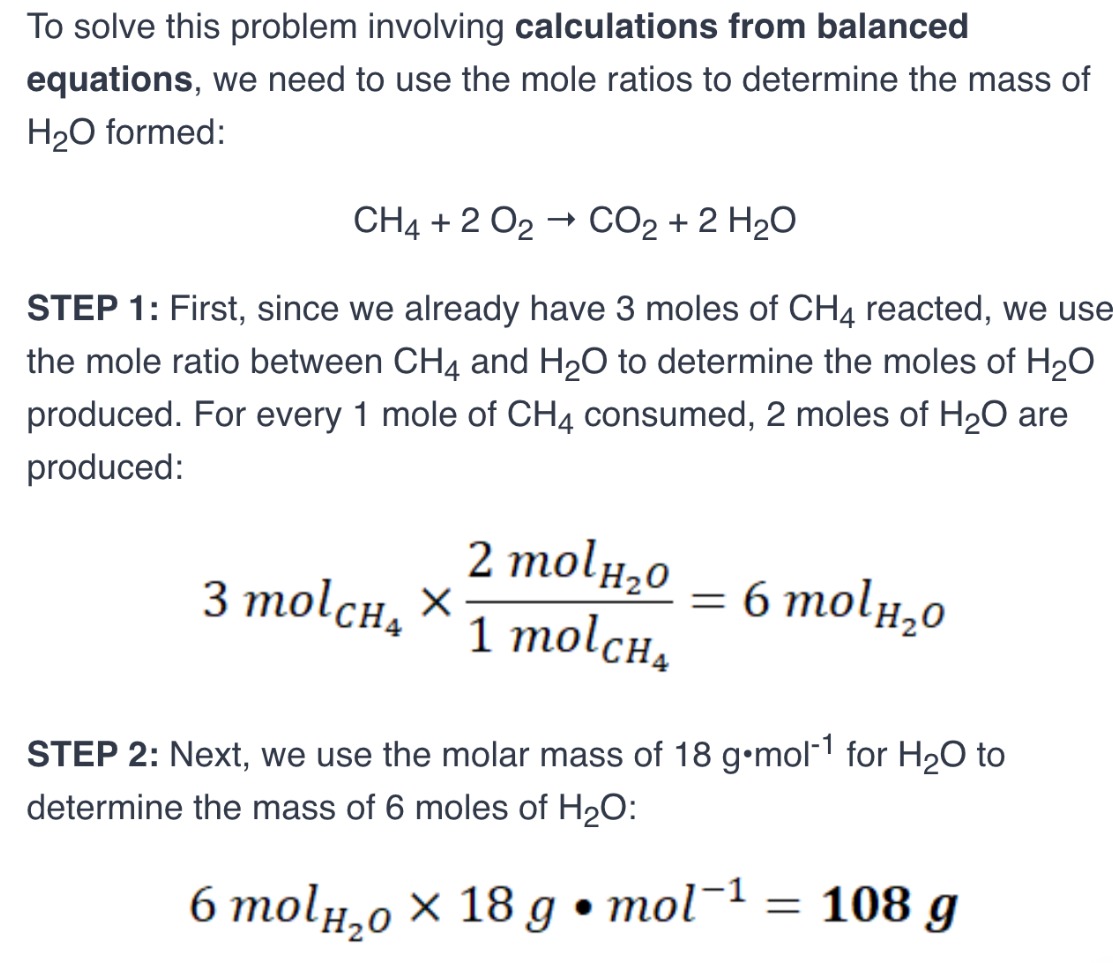
What is a limiting reagent?
It is the reactant that determines how much product is formed. It is completely used up in a reaction
What are the four steps to determine the limiting reagent?
Make sure the chemical equation is balanced
Convert all the given information to moles
Pick one of the reactants as the limiting reagent and determine how much of the other reactant is required for the reactant that you chose
If there is an excess of the other reactant, the reactant you chose is the limiting reagent. If there is a shortage of one of the reactants, the other reactant is the limiting reagent.

What is density?
A substance’s mass per unit of volume ( an intrinsic property that is specific for a certain substance)
What is the formula used to find density?
see picture

True or false: Under constant temperature and pressure, the density of a pure substance also remains constant
True, because density is an intrinsic property of the substance.
A cube has a side of 5 cm. It has a mass of 250 grams. What is density in g/cm3?
look at picture
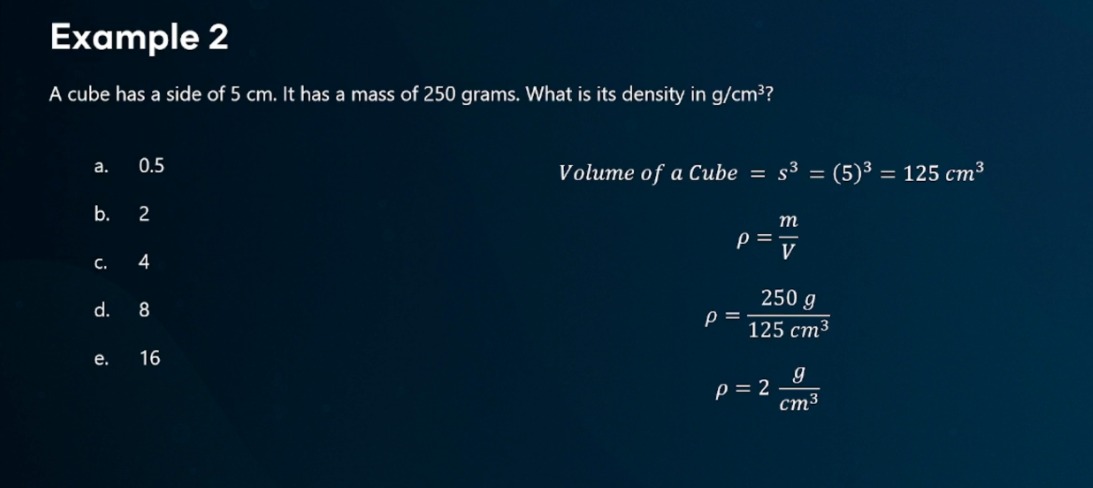
What is the actual yield?
The real quantity of a product resulting from a chemical reaction
What is theoretical yield?
The maximum number of products you can expect from an ideal chemical reaction,
What is percent yield?
The actual yield divided by the theoretical yield times 100%
What is the percent yield formula?

Theoretical yield is calculated using the?
limiting reagent