4C - Electrode potentials and cells
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
Whats an electrochemical cell made of?
Two different metals dipped in salt solutions of their own ions and connected by a wire and salt bridge (redox process)
How do electrons flow in an electrochemical cell?
From the most reactive metal to the least
What is the cell potential?
The voltage between the two half cells
What is need if the half cell only contains two aqueous ions?
Platinum electrode
What do electrochemical cells look like?
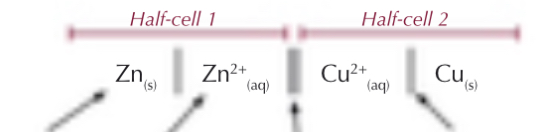
What electrode potential do you always put on the left?
Lowest (left lowest)
What is the equation for calculating the cell potential?
E0 cell = right hand side - left hand cell (more negative - more positive) (reduced - oxidised)
What is the anode?
Where oxidation occurs (gains electrons)
What is the cathode?
Where reduction occurs (loses electrons)
What format are electrode potentials always in?
Reduction reactions
What is the standard hydrogen electrode?
The electrode potential that all the others are compared to
What are the standard conditions for a hydrogen electrode?
Concentration of 1moldm-3
Temperature of 298K
Pressure of 100kPa
What is the electrochemical series?
List of electrode potential for different electrochemical half-cells
What does it mean if a reaction is not feasible?
It will not occur
When are some cells non-rechargeable?
Irreversible reactions used
What makes a cell rechargeable?
Reversible reactions
What is the overall reaction in a alkaline fuel cell?
2H+ + O2 → 2H2O
What is the reaction at the negative electrode of the alkaline fuel cell?
H2 + 2OH- → 2H20 + 2e-
What is the reaction at the positive electrode of the alkaline fuel cell?
O2 + 2H2O + 4e- → 4OH-
What are the pros of a fuel cell?
More efficient
Only waste product is water
Don’t need to be reacharged
What are the cons of a fuel cell?
Energy needed to supply hydrogen
Hydrogen is highly flammable
Infrastructure for hydrogen fuel cars doesn’t exist