chemistry moles
0.0(0)
Card Sorting
1/6
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
1
New cards
Avogadro’s number/constant
6.02 × 10 to the power of 23
2
New cards
how to calculate the mole of an element
atomic number x avogadro’s number
or atomic number g/mol
3
New cards
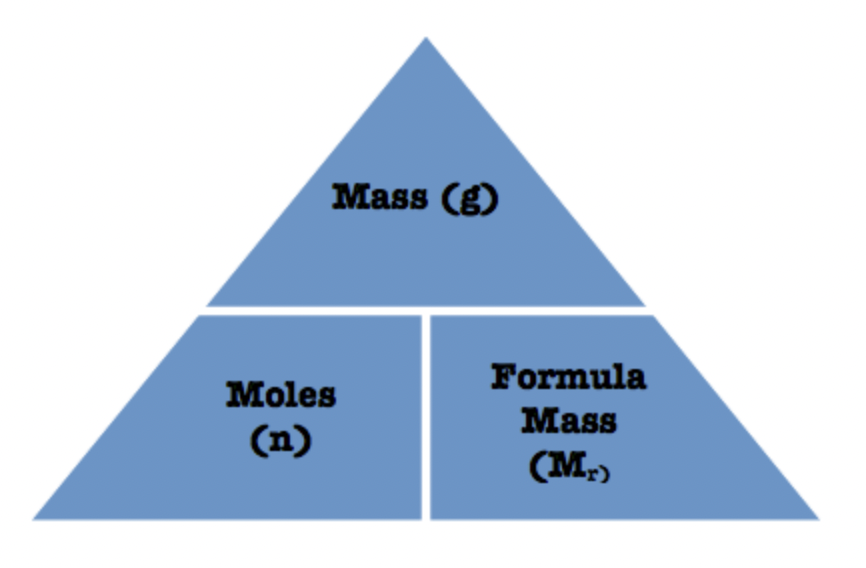
moles formula/triangle
m → grams, n → moles, M → molar mass
to find moles → mass/molar mass
to find grams → moles x molar mass
4
New cards
how to find the concentration
number of moles/volume
5
New cards
cm cubed to dm cubed
cm cubed/1000
6
New cards
how to find volume of a gas
Volume = moles of gas x 24dmcubed
7
New cards
what is a mole
the unit of the amount of substance