Introduction to Organic Chemistry: Alkanes
1/174
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
175 Terms
Organic compounds
Organic compounds must contain carbon which is covalently bonded to at least one other element
HIghlighted red are some exceptions
Hydrocarbon
Hydrocarbon is a molecule which only contains hydrogen and carbon
Saturated hydrocarbons
Saturated hydrocarbons contain only single carbon bonds. Saturated hydrocarbons are full which means they contain the maximum number of hydrogen atoms possible
Unsaturated hydrocarbons
Unsaturated hydrocarbons containing one double bond or triple bond. We call them unsaturated because these molecules aren’t filled with hydrogen atoms so the molecule isn't saturated
what are alkanes
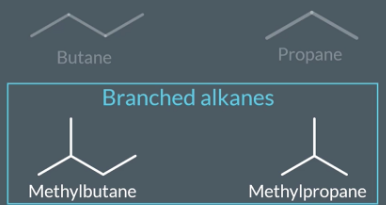
linear and branched alkanes
what are alkanes
branched
linear
is this an alkane
Even those these do contain single cabron-cabron bonds its in a ring shape so is not an alkane
what are cycloalkanes
"What are cycloalkanes, and how do they differ from alkanes?"
Cycloalkanes are saturated hydrocarbons meaning that they contain only carbon and hydrogen, and only single carbon-carbon bonds. But whereas alkanes are either linear or branched, cycloalkanes form rings
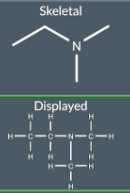
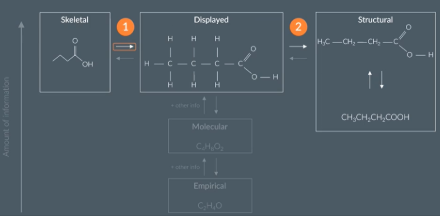
The different ways to represent molecules.
T
the different ways to represent molecules. Remember this as it will be useful when converting one formula into a different formula (e.g drawing a structural formula into a skeletal formula - for this we have to draw a displayed formula first)
"What does the molecular formula represent, and how is it written?"
The molecular formula gives the actual number of atoms of each element in a molecule
When we write the molecular formula for first we write the number of carbon atoms, then the number of hydrogen atoms, then all other atoms in alphabetical order.
So to find the molecular formula we first need to count the number of atoms in each element, list carbon and hydrogen first and then list the other elements not carbon and hydrogen in alphabetical order
what is the empirical formula
The empirical formula gives the simplest whole number ratio of elements in a compound
"What does a displayed formula show?"
A displayed formula displays the position of every atom in the molecule and also the bonds between them
"How are different types of bonds represented in a displayed formula?"
Each bond is represented with a line (so one line is equal to one bond). 2 lines combining from one element to another represents a double bond. 3 lines represent a triple bond.
how to draw C3H7Br in a displayed formula
In the question it states it is a linear molecule.
First draw the linear chain of carbons.
We know the bromine atom is at the end of the chain so add that in
We know there are 7 hydrogen atoms and in any molecule carbon forms 4 bonds so we fill in the hydrogen so that each carbon has 4 bonds
how to draw C3H7Br in a displayed formula
First draw the carbon atoms in a linear chain
Next fill in the hydrogen atoms
We know that in any molecule carbon forms 4 bonds but there are 2 carbon atoms with only 3 bonds so we add a double bond between them.
drawing the displayed formula
IN THE EXAM: if you're asked to draw the displayed formula of a molecule you must show every single atom and every single bond in a molecule otherwise you won't get the mark. In the above exam the CH2 bond hasn't been shown/displayed so you wont get the marks
when we draw a displayed formula …
SO when we draw a displayed formula first draw the non hydrogen atoms then fill in the hydrogen atoms and then check that each atoms has the right number of bonds
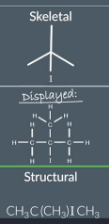
structural formula definition
identifying a structural formula
A structural formula doesn't display all the bonds in molecular unlike a displayed formula it does tell the position of the atoms in a molecule.
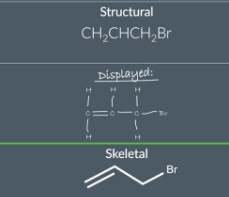
"How can the structural formula CH3CH2CH2Br be used to draw the displayed formula for the molecule?"
E.g For example if a molecule has the structural formula CH3CH2CH2Br we can say that the 1st carbon atom bonded to 3 hydrogen atoms the 2nd carbon atom is bonded to 2 hydrogen atoms and the 3rd carbon atom is bonded to 2 hydrogen atoms and 1 bromine atom. This gives us enough information to draw the displayed formula for the molecule.
"How are side chains represented in structural formulas
In structural formulas to represent a side chain we point it in brackets. In this example we know that the side chain is attached to the 2nd carbon atoms.
"What is the purpose of drawing out non-single bonds or side chains in a structural formula, and why are these still considered structural formulas?"
Sometimes you might see a structural formula which draws out any bonds that aren't single bonds or which draw out the side chains. Because these still give us the full information about the structure of the molecule without displaying ALL of the bonds we still can them structural formulas
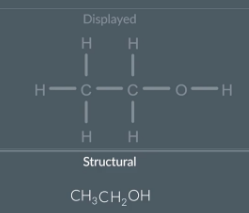
Drawing a structural formula from a displayed formula
First look at each non hydrogen atom in the displayed formula and collapse that atom plus the hydrogen bonded to it into one group
Next remove the bonds
Reorganise the symbols of each group so that the hydrogen atoms are at the end and squish them all together
This is the structural formula
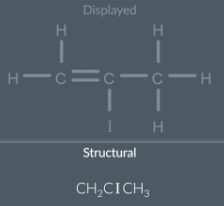
displayed to structural formula: double bonds
First look at each non hydrogen atom in the displayed formula and collapse that atom plus the hydrogen bonded to it into one group
Becucae halogens only form 1 bond, Iodine is only bonded to this carbon so we can also collapse the iodine atom into the group with this carbon
Remove the bonds
Reorganise the symbols of each group so that the hydrogen atoms are at the end and squish them all together
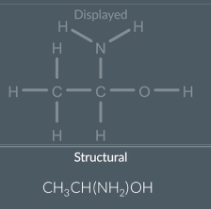
displayed to structural formula: side chains
First look at each non hydrogen atom in the displayed formula and collapse that atom plus the hydrogen bonded to it into one group
Next remove the bonds
Reorganise the symbols of each group so that the hydrogen atoms are at the end
We have side chain which contains a H2 so we need to write this in brackets after the carbon that is it bonded to
The squish this all together
drawing a structural formula
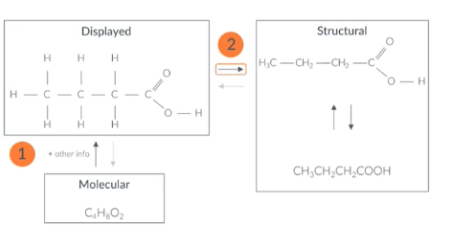
When finding the structural formula from the molecular formula
When finding the structural formula from the molecular formula first sketch out the displayed formula then turn that into the structural formula.
In the exam you won't be marked for the displayed formula so you don't have to draw a perfect on just draw the skeletal formula
Draw the displayed formula
Carbon forms 4 bonds, Bromine forms 1 bond Sulfur forms 2 bonds
Turn the displayed formula into a structural formula
To get the structural formula we put the atoms into groups remove the bonds.
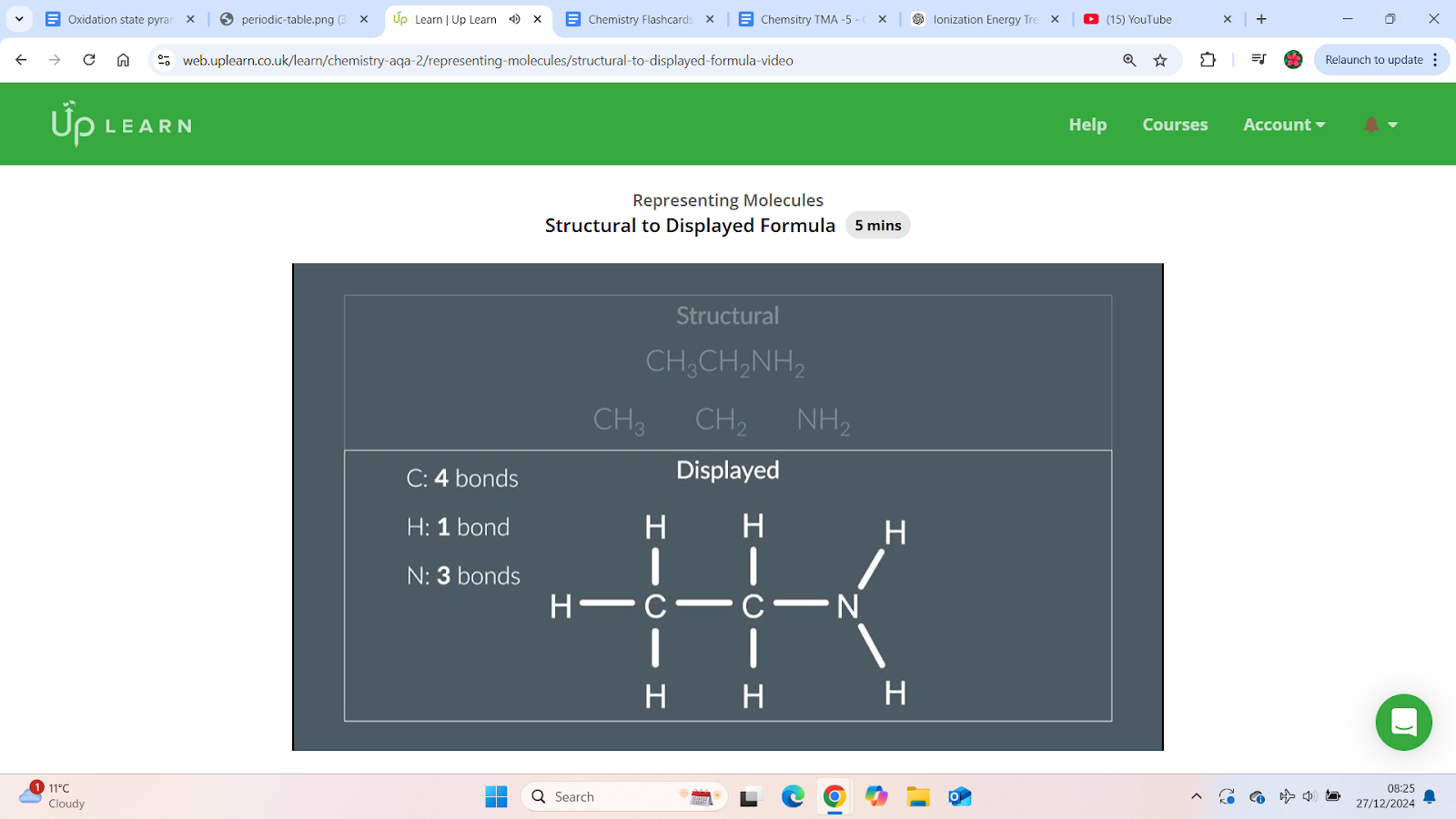
How to draw the displayed formula from the structural formula
Locating groups in a molecule
Locating groups in a molecule:
Identify each non-hydrogen atom and group it with the hydrogen atoms surrounding it.
Example:
First group: Carbon bonded to 3 hydrogens.
Second group: Carbon bonded to 2 hydrogens.
Continue for all atoms in the molecule.
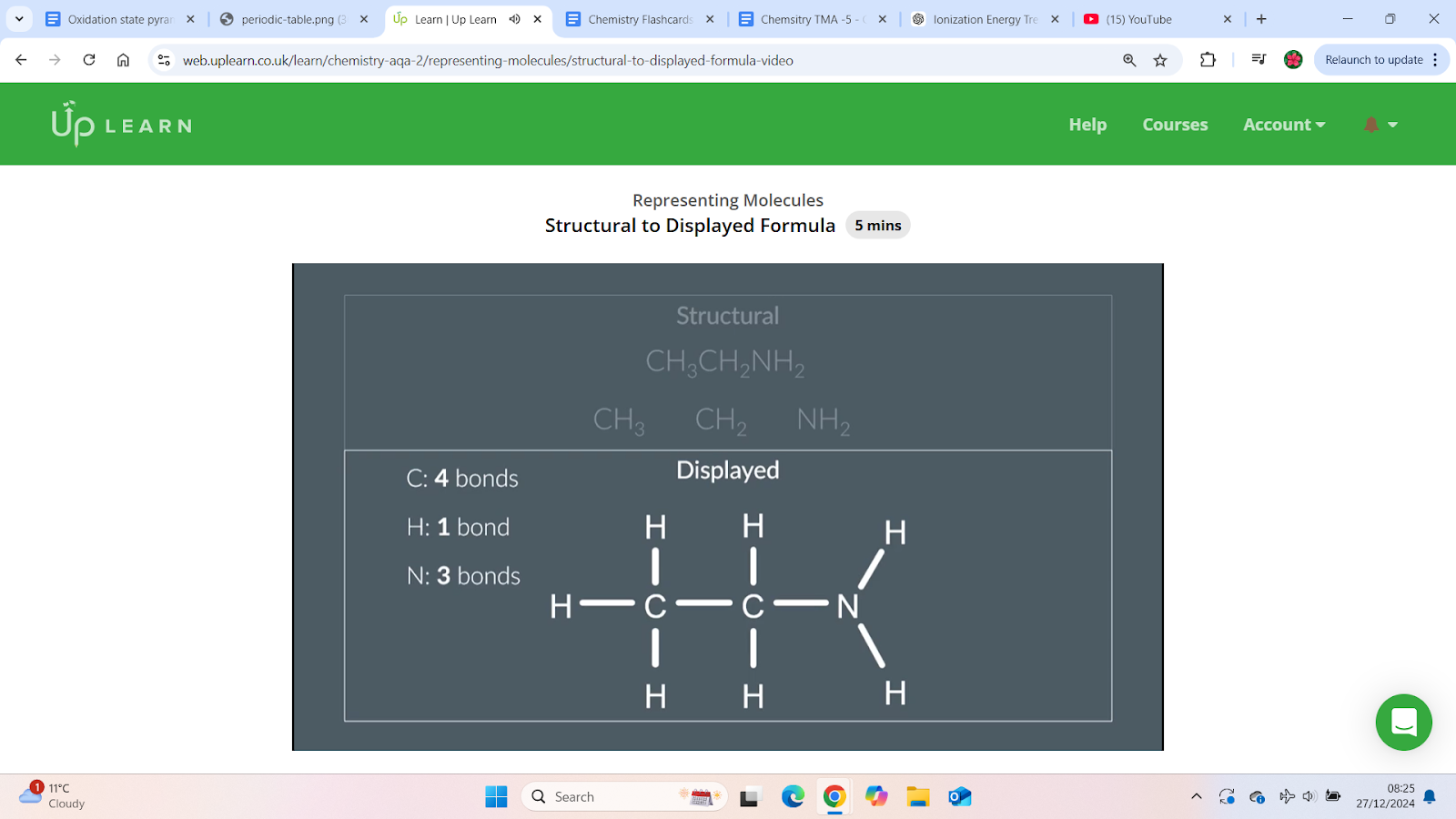
How to draw the displayed formula from the structural formula
Drawing the structure:
Drawing the structure:
Arrange carbon, carbon, and nitrogen in a linear fashion.
Use single bonds between them.
Add hydrogens around each atom as per the number of bond each atom can form .
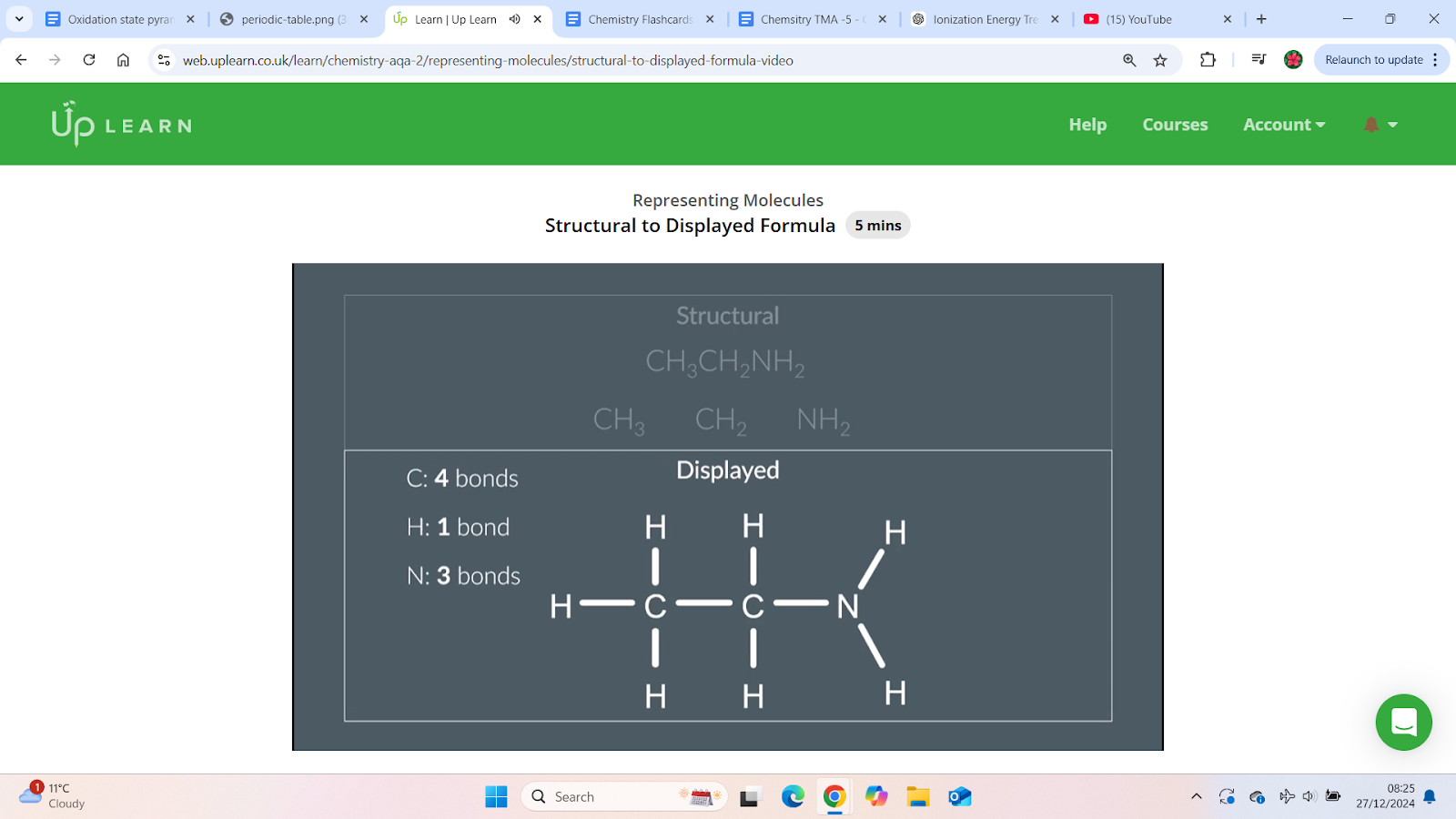
How to draw the displayed formula from the structural formula
Bond rules for atoms:
Carbon: Forms 4 bonds.
Hydrogen: Forms 1 bond.
Nitrogen: Forms 3 bonds.
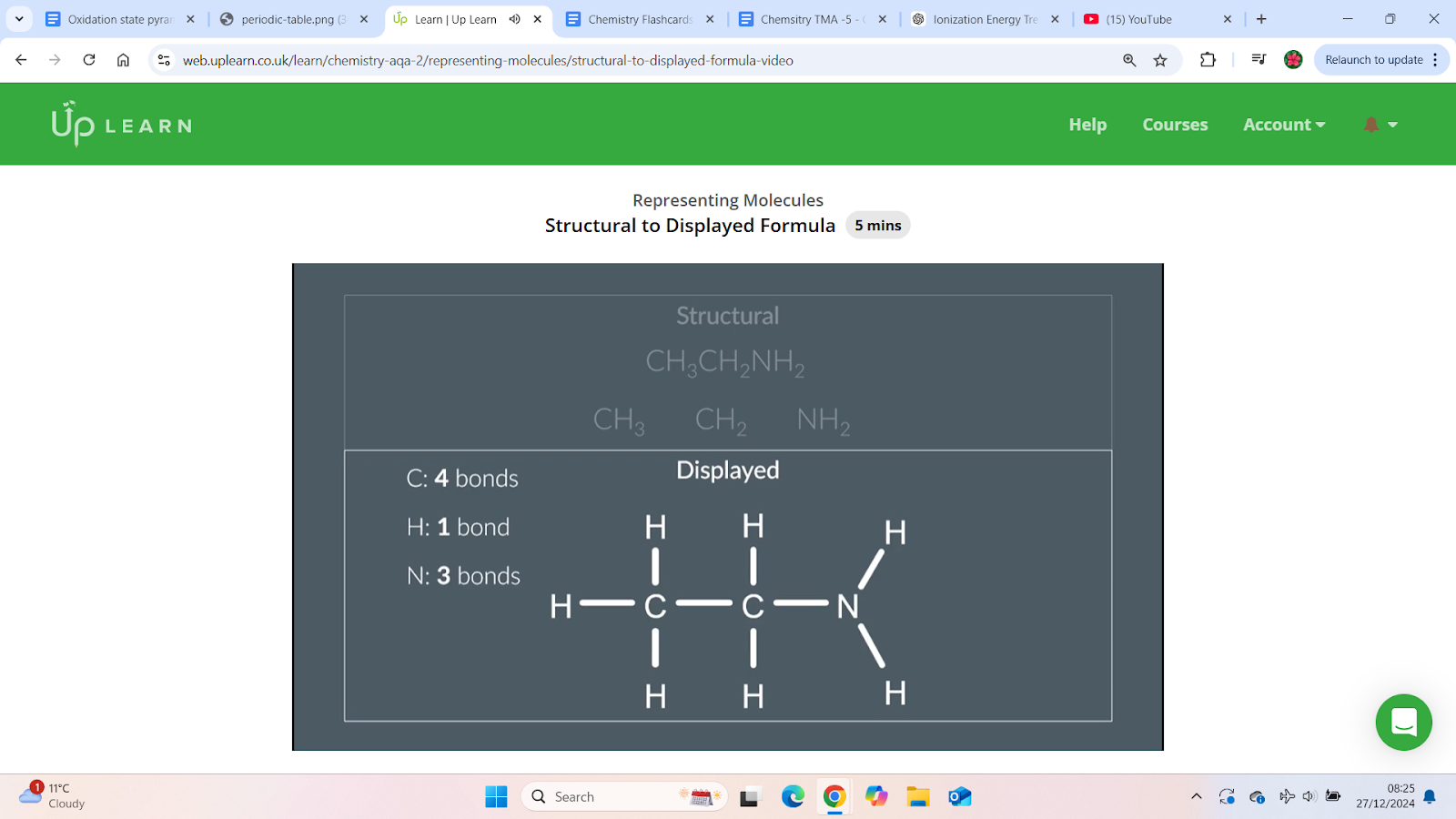
How to draw the displayed formula from the structural formula
Checking the displayed formula:
Count the number of bonds around each atom.
If there are insufficient bonds, add double or triple bonds as necessary.
In this example, no double/triple bonds are needed.
converting a structural formula into displayed formula
How to find the molecular formula from the structural formula
count up the number of atoms of each in element in a molecule
find the empirical formula from the structural formula
The empirical formula gives the simplest whole number ratio of all the elements in a compound
To find the empirical formula we first find the molecular formula and then calculate the empirical formula form that
finding the molecular formula from the structural formula
"What are the drawbacks of using displayed formulas?"
Take ages to draw out
Hard to interpret
"What are the advantages and limitations of using a skeletal formula?"
quicker to draw out compared to a displayed formula but still slow
Doesn’t show single, double or triple bonds
"What is the main limitation of molecular and empirical formulas?"
Good for showing which atoms are in the molecule
But do not tell is anything about the structure
what are skeletal formulas
Skeletal formulas just show the skeleton of the molecule
E.g in this molecule there are 4 carbon atoms and each cabron-cabron bond is a single bond. Four lines are used to show this. The first carbon cabron bond is highlighted above
In a skeletal formula each carbon atom is displayed at the end of each bond (coloiured dots show the carbon atoms)
"What is a skeletal formula, and how does it differ from a displayed formula?"
No hydrogen should be shown.
In a displayed formula we show the carbon atoms and the cabron hydrogen bonds. IN a skeletal formula we dont show these because we know that carbon forms 4 bonds and so we just show carbon bonds to atoms that aren't hydrogen and assume that hydrogen make up the remaining bonds
E.g on the 1st carbon atom we can only see 1 bond to another carbon so we know that there are 3 hydrogen atoms also bonded to this carbon. In the 2nd carbon atom we can see 2 bond so we know that there are 2 hydrogen atoms also bonded to this carobn
skeletal formula definition
And they do this by showing all the carbon carbon bonds in a molecule but they don't show any carbon hydrogen bonds or any of the carbon or hydrogen atoms
list of skeletal formulas: what they have and what they don’t
drawing skeletons: non hydrocarbons
We don't write any Cs or Hs but we do write At (non C or H atom) to show the astatine atom and we show the carbon-astatine single bond (non C/H atom—- C single bond)
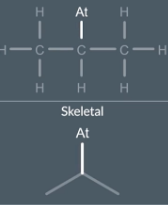
drawing skeletons: non hydrocarbons and double bonds
We don’t write any Cs or Hs but we do write Oto show the oxygen atom and we show the carbon-oxygen double bond
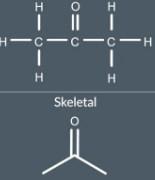
"When do we write hydrogen atoms in a skeletal formula?"
We don't write any Cs or Hs but we do write N for the nitrogen atom, ANd unlike hydrogen atoms attached to carbon atoms IF there are hydrogen atoms attracted to any NON-CARBON atoms then we do write this i although we don’t have to show the bonds. OS we write NH2 to dhow the NH2 atom and we show single bond between NH2 and carbon
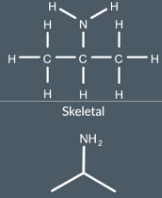
skeletal formulas for non-hydrocarbon molecules show…
"In a skeletal formula, what do the lines and points represent? How can you determine the number of carbon atoms?"
Remember when drawing skeletal formulas the line does not represent the carbons atoms. The points represent the carbon atoms, Though there are 3 lines in this diagrams it has 4 point which represent 4 carbon atoms.
A good equation to remember
Number of carbon atoms - 1 = Number of lines drawn ONLY USE FORMULA FOR LINEAR HYDROCARBONS (ZIGZAG)
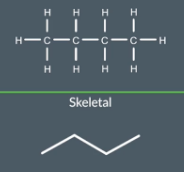
"How do you represent a C=C double bond in a skeletal formula?"
In this diagram there is still 4 carbon atoms but we add a double bond to one of lines to show C=C double bond.
Count the carbon atoms and draw the lines. (numebr of carbon atoms - 1 = number of lines drawn)
Draw the double bond in in a zigag
"How do you represent a C≡C triple bond in a skeletal formula?"
We draw triple bond linearly
Count the carbon atoms and draw the lines. (numebr of carbon atoms - 1 = number of lines drawn). (4 carbon atoms so 3 lines) drawn linearly
Add the triple bond in linearly
"How do you draw a skeletal formula with 5 carbon atoms and a double bond between the first two?"
There are 5 carbon atoms so 4 lines
Add double bond to 1st line between 1st 2 carbon atoms
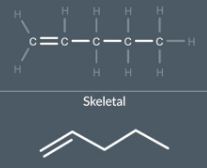
"How do we draw single, double, and triple bonds in a skeletal formula?"
When we draw skeletal formulas we draw single and double bonds in a zigzag. We draw triple bonds linearly
"How do we represent branches in a skeletal formula which are carbon and hydrogne ?"
When we have a branched molecule we just draw the side chain in the same way that we draw the rest of the skeletal formula.
"What is a cyclic molecule?"
When a molecular contains a ring we call it a cyclic molecule
"How do we represent a molecule with 4 carbon atoms in a ring in a skeletal formula?"
This molecular has 4 carbon in a ring so we draw a square (as all lines should be straight not bent) with each point representing a carbon atom
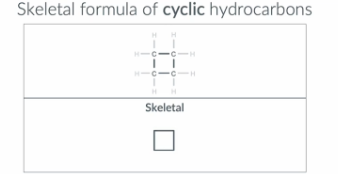
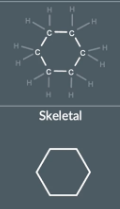
"How do we represent a molecule with 6 carbon atoms in a ring in a skeletal formula?"
This molecule has 6 carbon in a ring so we draw a hexagon with 6 point to represent each carbon
how to draw the skeletal formula: non hydrocarbons
How to draw a skeletal formula from a structural formula
To draw a skeletcal formula from a strucutrual formula first we have to draw the displayed formula.
how to turn a skeletal formula into a displayed formula
We draw the carbons. There are 4 points (ignore N) so 4 carbon atoms all joined by single bonds.
There is a nitrogen in between the 2nd carbon and the 3rd carbon so we draw a horizontal line C-C-N-C
The 4th carbon atom is attached to the N atom in a side chain so draw that in
Next we know that carbon makes 4 bonds so fill in the hydrogen atoms.
when we convert a skeletal to a displayed formula we
To draw a structural formula from a skeletal formula
To draw a structural formula from a skeletal formula we first draw the displayed formula anf then draw the structural formula
To get the structural formula we group the atoms up and then rearrange so that the hydrogen are are at the end. Then we put the groups in one line and smush them together. REMEMBER to put any side chain in brackets
To draw a molecular formula from a skeletal formula
To draw a molecular formula from a skeletal formula first we draw the displayed formula and then we calculate the molecular formula
We calculator the molecular formula by counting the number of atoms of each element in a molecule
calculating the empirical formula from the molecular formula
We can also work out the empirical formula by simplifying the molecular formula to the simplest whole number ratio
what does the question ‘give the IUPAC nomenclature?’ mean
GIve the name of the molecules according to the IUPAC rules for naming molecules
Nomenclature
- process of naming molecules
what does IUPAC stand for
IUPAC- international union of pure and applied chemistry
"How do we name an alkane with two carbon atoms?"
Whenever we are naming a molecule which is an alkane we always end the name with ‘ane’
The prefix ‘eth’ tell us that their are exactly 2 carbons in this alkane chain
prefix for the number of carbons
Pent = 5 because a pentagon has 5 side
Hex = 6 because a hexagon has 6 sides
"How do we name branched alkanes?"
When we name branched alkanes we name the ,longest chain like a linear alkane and we name the side chain with prefix (meth, eth, prop). The prefix should tell us how many carbon are in the side chain. We add ‘yl’ to the end of the prefix
SO
Number of carbons in side chain ’yl’ longest linear chain
Name this alkane
Number of carbons in side chain ’yl’ longest linear chian
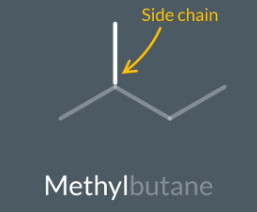
Name this alkane
5 carbons in longest chain (so pentane)
2 carbons in side chain so eth
Add yl
Eth yl pentane
Ethylpentane
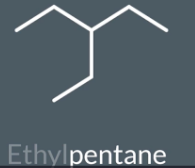
How to find the longest carbon chain
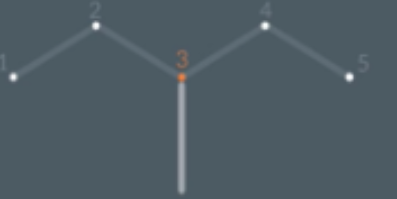
"How do we indicate the position of a side chain in a branched alkane's name?"
We need to be able to differentiate between two molecules that have the same name but have different positioned side chains
To do this we label each carbon in the longest chain and see which number carbon the side chain is attached to. Then we put this position number in font of the prefix/name(e.g methyl) with a dash between the two.
We call this number the position number and for A levels we always include the position number in the name of the molecule
Position number - name
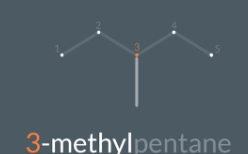
"How do we choose the correct position number when naming a branched alkane?"
We always name the carbon based on which gives the lowest position number so 3 gives the lowest position number so we call it 3-ethyheexane
What does the position number tell us
The position number in a molecules name tells us where the side chain is located on the longest chain
Steps to Draw an Alkane from Its Name:
Draw propane
Identify the longest chain – The prefix (e.g., "prop-") tells you the number of carbon atoms. Draw them in a straight line.
Determine the bond type – The suffix ("-ane") indicates single bonds. Connect the carbon atoms with single lines (C-C-C).
Add hydrogen atoms – Ensure each carbon forms 4 bonds by adding hydrogen atoms where needed.
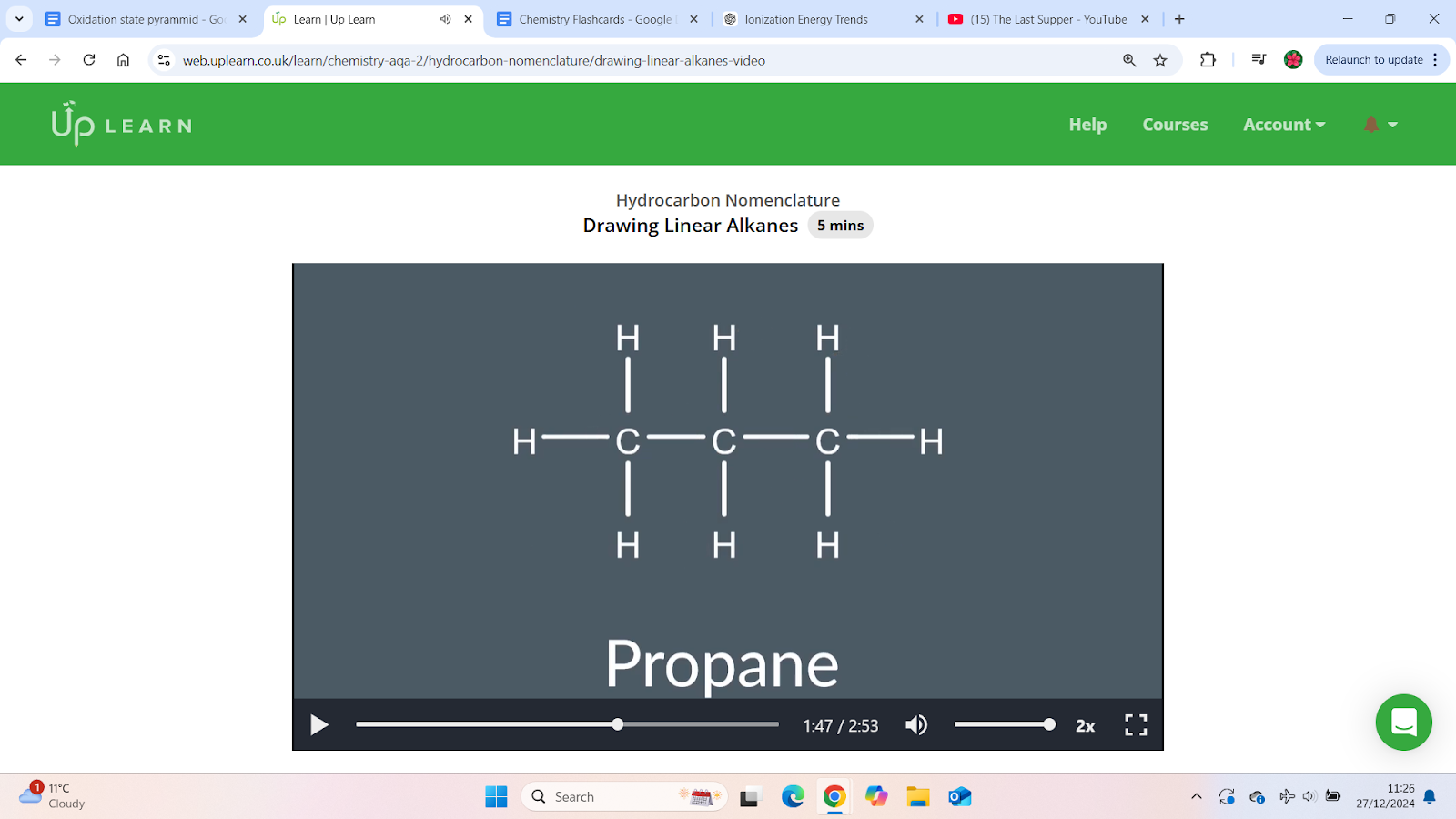
When drawing a linear alkane
Look at how many carbon are in the chain and draw them in a row
Next draw single bonds between the carbon
Then fill in the hydrogen to make sure each carbon forms 4 bonds
Steps to Draw a Branched Alkane:
"How do you draw the structure of 3-ethylhexane?"
Draw the longest chain – Identify the main chain from the name (e.g., "hexane" = 6 carbons) and connect them with single bonds.
Identify the side chain – Look at the prefix (e.g., "3-ethyl" = 2-carbon side chain).
Number the main chain – Label the carbons to find the correct attachment point for the side chain.
Attach the side chain – Draw the side chain at the specified position using single bonds.
Add hydrogen atoms – Ensure each carbon forms 4 bonds by filling in hydrogens.
drawing branched alkanes
First draw the carbon in the longest chain
Connect them with single bonds
Draw the side chain on the correct carbon on the longest chain
Connect them with single bonds
FINALLY FILL IN THE HYDROGENS so that each carbon has 4 bonds in total
"How do we name cycloalkanes?"
When we are naming cycloalkanes we name them like we would a linear alkane and add ‘cyclo’ to the front
"How do you name a cycloalkane with 4 carbon atoms?"
This molecule contains four carbon atoms with only single bonds so we write butane. These 4u carbon atoms are in a ring so we add cyclo to the front. Therefore it is called cyclobutane
"Why don’t cyclomethane and cycloethane exist?"
Cyclopropane is the smallest possible cycloalkane. Cyclomethane and cycloethane don't exist because we cant form rings out of only 1 or 2 carbons
"What determines the side chain in a cycloalkane?"
The side chain doesn't necessarily have to be the shortest chain it just need to be the chain outside of the cycloalkane
"How do we name a cycloalkane with one side chain?"
When a cycloalkane has one side chain we don't need to specify which carbon the side chain is on.
Name the ring like a linear alkane (butane, propane etc depending on the number of carbon in that ring)
Then add cyclo to the beginning of it.
Count the number of carbon on the side chain and add the prefix +yl to the beginning of the word.
draw cyclobutane
Cylco shows that it is in a ring. So draw the carbon a ring and connect the with single bonds
NO prefix before cyclo so there's no side chains. Instead we just fill in the hydrogen so each carbon has 4 bonds in total
"How do you name and draw a cycloalkane with a side chain?"
Identify the ring type from the suffix (e.g., "butane" = 4 carbons in a ring).
Draw the ring with single bonds between the carbons.
Look at the prefix (e.g., "prop-" = 3-carbon side chain).
Attach the side chain to any carbon in the ring.
Fill in the hydrogens to ensure each carbon has 4 bonds
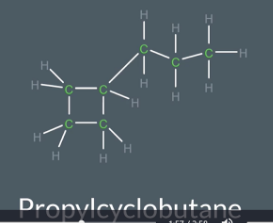
steps to draw a cycloalkane
Draw the number of cabron in the ring
Connect these carbons with single bonds
If there is a side chian draw attached to any carbon in a ring and connect them all with single bond
Fill in the hydrogen so that each carbon has 4 bonds in total
what is a functional group
A functional group is a group of atoms in a molecule which determines how the molecule reacts with other substances
Any group of atoms that isn't a saturated carbon chain or ring are functional groups
"What are the names of these functional groups: OH, NH2, and C=C?"
Each functional group has its own name
OH is an Alcohol group
NH2 is an Amine group
C=C is an Alkene group
A homologous series is a group of compounds that
same functional group
different number of CH2 groups
chemically similar
Gradual change in physical properties
Same general formula
"What is a homologous series and how do compounds within it differ?"
In a homologous series even though all compound have the same functional group each compound might contain a different number of saturated carbon atoms:E.G this group all have the same functional group but different number of staturedt cabron atoms (CH2)
"How does the functional group affect the reactivity of a molecule?"
The functional group determines the molecule reactivity
If different compound have the same functional group then they will still all react in the same way/ are chemically similar
"How do compounds in a homologous series behave chemically?"
In a homologous series all the compounds have the same functional group meaning that they react in the same way and so are chemically similar
"Why are alkanes and cycloalkanes chemically similar and unreactive?"
Alkanes and cycloalkanes don't have fucnitonal groups as they are only made of saturated carbon chains and this means they are extremely unreactive. IN each of these homologous series all of these compoiund are unreactive so we can still say that they are chemicallhy similar
"How do longer carbon chains affect intermolecular forces and physical properties?"
Longer carbon chains have stronger intermolecular forces due to more electrons, leading to stronger temporary dipoles. Intermolecular forces are responsible for physical properties like melting point, boiling point, and solubility.
"How does chain length affect boiling points in a homologous series?"
In a homologous series, longer carbon chains with more CH₂ groups increase electrons, strengthening van der Waals forces and boiling points
"How does adding CH₂ groups affect alkanes' melting and boiling points?"
For example, in the alkane homologous series, as we move from methane to ethane to propane to butane, each step involves adding a CH₂ group, increasing the carbon chain length and the number of electrons. This results in a gradual increase in melting and boiling points.
"How do physical properties change as CH₂ groups increase in a homologous series?"
As the number of CH₂ groups increases in a homologous series, the physical properties of each compound also gradually increase—slightly but consistently between consecutive compounds.