Module 2: Enzymes
1/75
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
76 Terms
What are enzymes -
They are biological catalysts and they're globular proteins
What is metabolism? -
The sum of all chemical reactions within cells and organisms
What is an anabolic reaction? -
One which builds a molecule or molecules
What is a catabolic reaction? -
One which breaks down a molecule or molecules
Where do intracellular enzymes act? -
Inside cells
Where do extracellular enzymes act? -
Outside of cells
Why are extracellular enzymes needed? -
Nutrients in the form of polymers need to be broken down
What do single celled organisms do with extracellular enzymes -
They release them into their immediate environment
What is the structure of catalase -
A conjugated globular protein with 4 units, a heme group in its quaternary structure.
Is catalyse intracellular or extracellular? -
Intracellular
What is the function of catalase? -
It breaks down hydrogen peroxide into water and oxygen
Is catalyse found in plants and animal tissues -
Yes
Where is amylase present, and is it intra or extracellular? -
Produced by saliva glands and pamcreas, extracellular
What is the function of amylase? -
Breaks down starch into maltose. Maltos is broken down into glucose (maltase small intestine)
What is the function of trypsin? -
It is a protease, and therefore breaks down proteins into peptides which can then be broken down to amino acids by other proteases
Where does trypsin work and is it intra or extracellular? -
Works in the small intestine, extracellular
What is the lock and key hypothesis? -
The substrate randomly moves into the enzyme's active site, which it is an exactly complementary fit for, forming an enzyme-substrate complex. The substrate is placed under stress through interaction with R-groups from the active site, breaking it/joining 2 substrates together
How do enzymes lower the activation energy of anabolic reaction? -
The tight fit between the two substrates in the enzyme-substrate complex reduces the repulsion between the two molecules, so they bond more easily
How do enzymes lower the activation energy of catabolic reactions? -
The strain placed on the substrate by its fitting in the active site causes it to break down more easily
How are EPC made? -
The reaction that occurs in ESC forms them
What is the induced fit theory? -
The substrate enters the active site of the enzyme, which is not complementary but a slightly different shape to the substrate. The active site slightly changes shape, putting pressure on the substrate via its R-groups and causing the reaction.
How does a low or high pH denature enzymes? -
The H+ or OH- ions affect the hydrogen and ionic bonding of the active site's tertiary structure, causing it to change shape
Can the enzyme return to its original shape after being denatured by pH? -
Yes, but only if pH changes slightly and then returns to optimum
What is denaturation? -
The loss of biological activitity
What is renaturation? -
The regaining of biological activity
How does temperature affect enzyme activity? -
At first, increased temperature increases kinetic energy for the enzyme's particles, so particles collide faster and more frequently, up to an optimum temperature.
What happens to enzymes if the temperature is too high -
After this, the enzymes particles have too much energy which begins to affect the bonds in its tertiary structure, making them vibrate and break apart changing the shape of the tertiary structure. Enzyme is denatured.
What is the optimum temperature for most enzymes? -
Around 40C
What adaptations do metabolic enzymes of animals living in cold conditions have? -
More flexible structures at the active site which makes them less stable than enzymes working at higher temperatures. Smaller temperature changes will denature them.
What adaptations do metabolic enzymes of thermophiles have? -
Enzymes are more stable than others due to the increased number of bonds-H+ and sulfur bridges in their tertiary structures. Shapes and active sites are more resistant to change as temperature rises
What is a Q10 value? -
How much the rate of reaction increases if the temperature of a reaction is raised by 10C. I.e. a Q10 value of 2 would mean the rate of reaction doubles.
What is Vmax? -
The maximum rate of reaction for a reaction
What Q10 value do most enzymes have? -
2
What is the trend of enzyme activity with varying pH? -
The enzyme activity increases up to the enzyme's optimum pH, after which it falls again, eventually reaching 0 at a high enough pH.
What is the trend of enzyme activity with increasing substrate concentration? -
The enzyme activity steadily rises for a period, after which the increase slows and eventually stops as Vmax is reached and all enzymes have a substrate molecule in their active site
Why do enzymes need regulating? -
So that products do not build up too fast and substrates are not used up too quickly
Investigation leading to different factors on enzyme activity -
Investigation of catalyse breaking down hydrogen peroxide into water and oxygen
Serial dilutions -


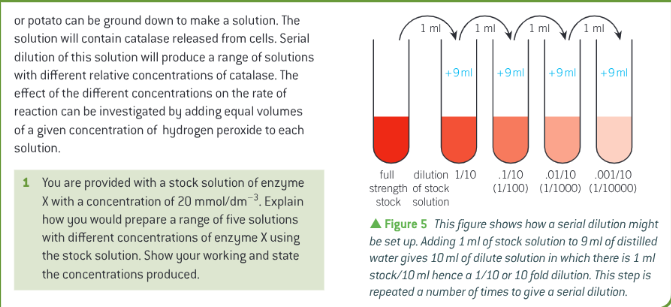

How can enzymes be activated or inactivated? -
Activated: Cofactors, Coenzymes and Prosthetic groups. Inactivated by inhibitors
How does a competitive inhibitor work? -
It enters the active site of the enzyme, preventing the substrate reaching the active site. No reaction takes place.
What is the structure of a competitive inhibitor? -
Similar (but not identical) to the structure of the substrate it stops being catalysed
How do Competitve inhibitors slow down the rate of reaction? -
Substrate and inhibitor present in the solution will compete with each other to bind to the AS of enzymes catalysing the reaction. Reduces the number of substrate molecules binding to the AS in a given time and slows down ROR.
How long do competitive inhibitors bind to the active site for? -
Most bindnonly temporarily so their effect is reversible. Some exceptions such as aspirin.
What are 2 examples of competitive inhibitors? -
Aspirin irreversibly inhibits active site of COX Enzymes, preventings synthesis of prostaglandins and thromboxane the chemicals responsible for pain and fever. Statins are competitive inhibitors of enzymes that synthesise chloresterol.
How is the Vmax affected by competitive inhibitors? -
It stays the same, but takes longer to reach
How do non-competitive inhibitors work? -
They bind to an allosteric site on the enzyme. This causes the enzyme, and its active site, to change shape, meaning that the substrate cannot bind to the enzyme as it's not complementary to the substrate
What is an allosteric site? -
A site elsewhere on the enzyme that is not its active site, to which a non-competitive inhibitor binds
How does increasing enzyme or substrate concentration affect the rate of reaction when non-competitive inhibitors are involved? -
Neither would affect it.
What are 2 examples of non-competitive inhibitors? -
Organophosphates and Proton Pump Inhibitors
What is an example of an irreversible non-competitive inhibitor? -
Organophosphates used as insecticides and herbicides
How do organophosphates work and what are they used for? -
They block an enzyme necessary in nerve impulses, and can thus cause cramp, paralysis and death. Used in insecticides and nerve agents such as Sarin, non-reversible
What enzyme do organophosphates irreversibly inhibit? -
Acetyl cholinesterase
What is the function of acetyl cholinesterase? -
It is necessary for nerve impulse transmission.
What are the effects of organophosphate inhibition of acetyl cholinesterase? -
Leads to paralysis and muscle cramps.
What type of inhibitor is a proton pump -
Irreversible non-competitive inhibitors
How are they irreversible inhibitors (PPI) -
Irreversibly block an enzyme responsible for secreting hydrogen ions into the stomtch. Causing long term indigestion
WHy do PPI lead to stomtch ulcers -
They reduce the production of excess acid in the stomach
How do non-competitive inhibitors affect Vmax? -
They lower it
What is end product inhibition -
the term used for enzyme inhibition that occurs when the product of a reaction acts as an inhibitor to the enzyme that produces it
How does End product inhibition serve as anegative feedback mechanism - Excess products aren't made or wasted. AN example of non competitive reverse inhibition
Excess products aren't made or wasted. An example of non competitive reverse inhibition
How is glucose broken down in respiration -
Addition of 2 phosphate groups.
What enzyme catalyses the addition of the second phosphate group which aids in the breakdown of glucose? -
Phosphofructokinase (PFK)
What inhibits PFK
ATP
What happens when ATP levels are high? -
More ATP binds onto the allosteric site of PFK. Prevents addiof the 2nd phosphate group to glucose. Glucose isn't broken down and ATP production is lower.
What happens as ATP is used up? -
Lesson it binds to PFK. PFK Is abel to catalyse the addition of the 2nd PO4 3- to glucose. Leading to more ATP production.
What is a cofactor? -
A non-protein component that is necessary for effective functioning of an enzyme
What are some common cofactors? -
Iron, zinc, calcium and chlorine (found in amylase) ions. Inorganic
What is a coenzyme? -
An organic cofactor, usually derived from vitamins.
What is a prosthetic group in the context of enzymes? -
A cofactor which is a permanent feature of the enzyme and essential to its function.
What is a precursor enzyme? -
An enzyme created in an inactive state and activated later on, for example digestive enzymes
How are Precursor enzymes activated -
Change in tertiary structure shape (active site). Can be achieved through the addition of a cofactor
What is the term for an inactive enzyme? -
Apoenzyme
What is a holoenzyme? -
An active enzyme since the cofactor is added.
What is a zymogen? -
An apoenzyme chose change into a holoenzyme is brought about by pH or temperature change or another enzyme
What is an example of a zymogen? -
Pepsin
What happens when inactive pepsinogen is released to the stomach to digest proteins -
The acid pH brings transformation into the active pepsin. This adaptation protect the body tissues against the digestive action of pepsin