Oxygen Binding (General)
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
35 Terms
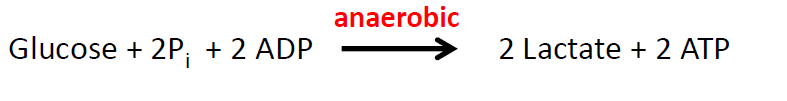
Equation for anaerobic respiration
Equation for aerboic respiration
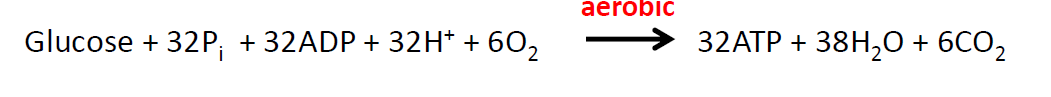
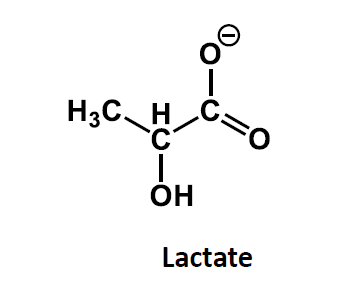
Structure of lactate
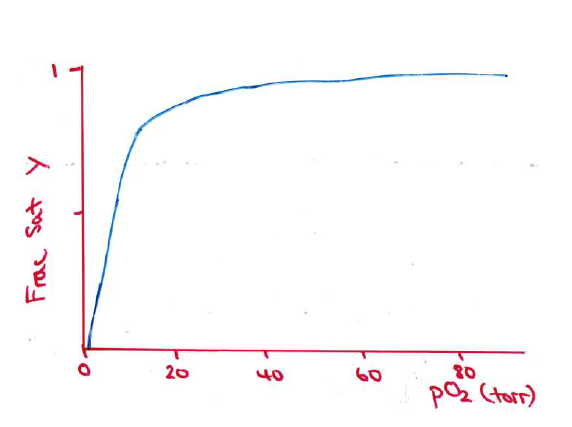
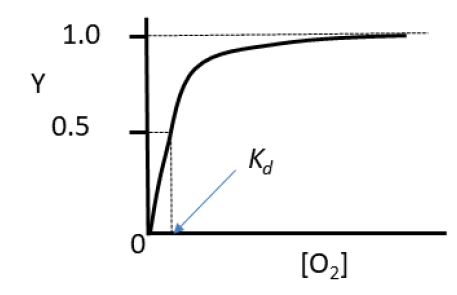
Oxygen Dissociation curve for myoglobin
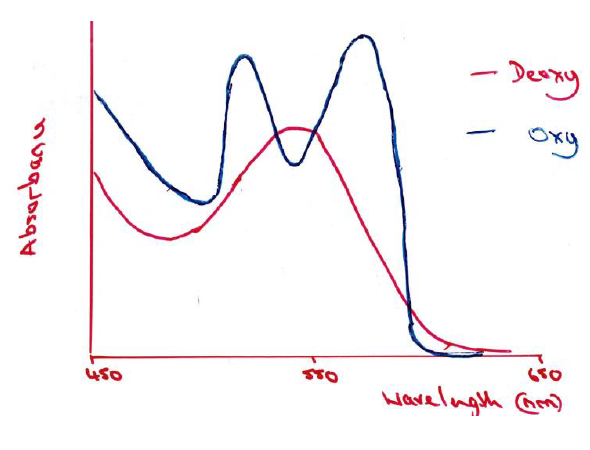
Absorption spectrum for deoxy and oxy myoglobin
Initial rate equation for myoglobin oxygen binding
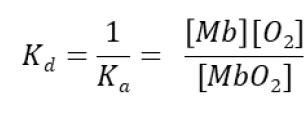
Rearranged rate equation for myoglobin oxygen binding
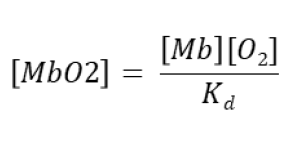
Equation for fraction of occupied oxygen binding sites
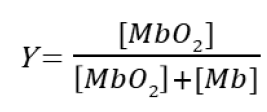
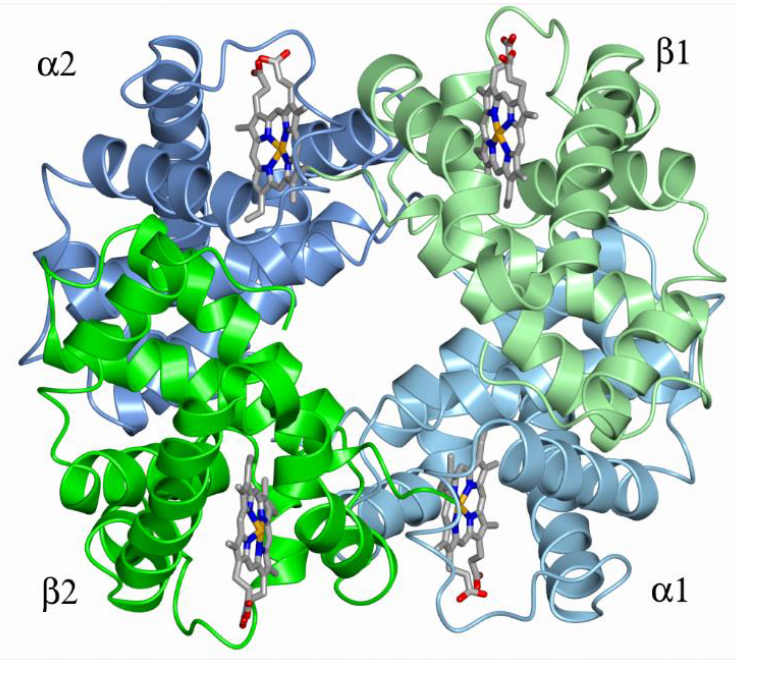
Plot of fraction of binding sites versus oxygen concentration
Ligand properties of oxy-myoglobin
Ligand properties of deoxy-myoglobin
Why is myoglobin used over free ferrous heme
Free heme can bind O2 but the Fe 2+ is rapidly oxidised to Fe 3+ and ferric heme no longer binds O2 reversibly
What does the polypeptide chain in myoglobin allow for?
Prevents dimerization to the oxygen bridge intermediate
Confers special chemical properties on the heme / protein interior is analogous to an organic solvent environment
Transfer coefficient ratio in free ferrous heme
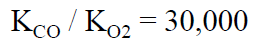
Transfer coefficient ratio in myoglobin
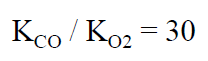
Why is the oxygen transfer coefficient for myoglobin higher?
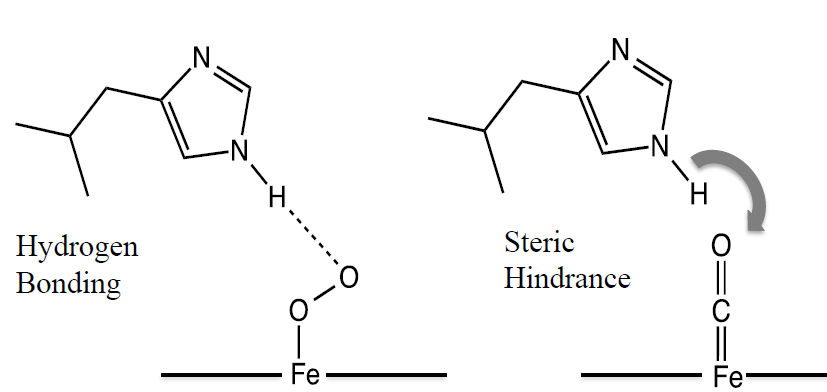
In myoglobin, hydrogen bonding with histidine interferes with bonded oxygen, allowing it to be broken off easier
Steric hindrance from the double bond prevents the same from occuring for CO
What mutation can reduce efficacy of myoglobin
Distal Histidine mutation Mb(ValE7)
Which histidine is replaced in Mb(ValE7)
His64
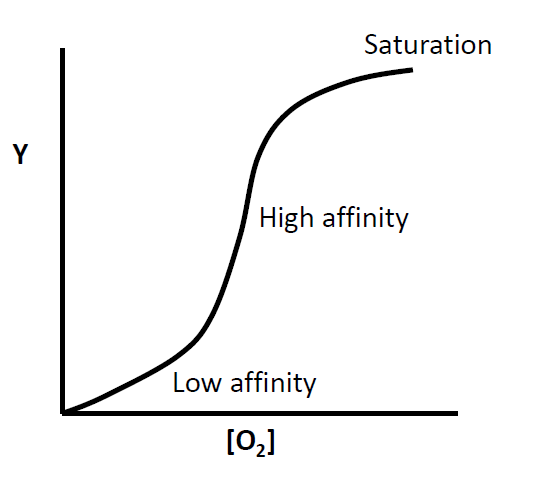
Transfer coefficient ratio in Mb(ValE7)
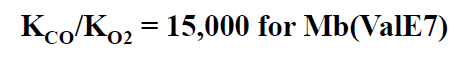
Oxygen association curve for myoglobin and hemoglobin
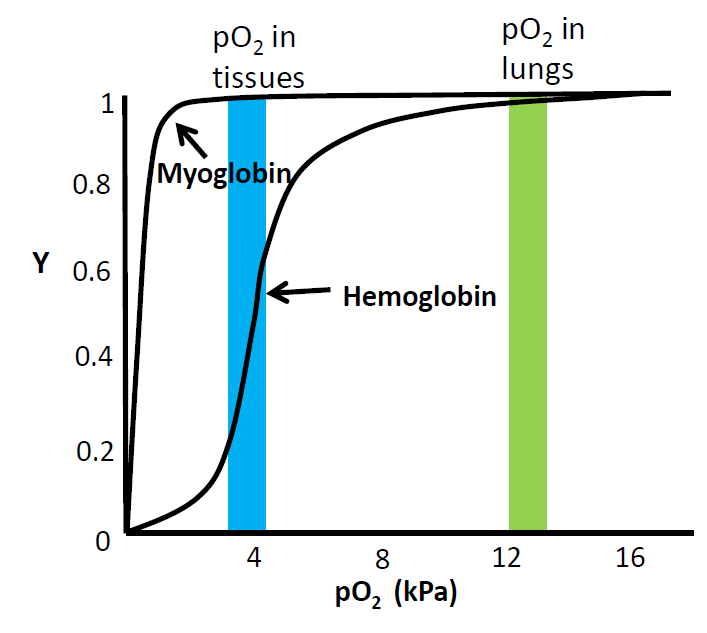
Structure of hemoglobin
How many subunits in hemoglobin

How many residues in either subunit type

Between which subunits are interactions strongest

Oxygen saturation curve
Hemoglobin binding equation(s)
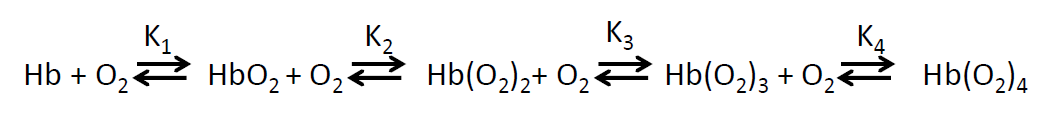
What does the sigmoidal saturation curve suggest about hemoglobin
K4 must be much bigger than K1
Thus oxygen binding is cooperative