Dissociation constants
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
Ka general information:
Ka of weak acids: Very small, Ka << 1
Ka of strong acids: Very large, Ka >> 1
Reason for difference in Ka of strong and weak acids: Strong acids completely ionise in solution, but weak bases don’t.
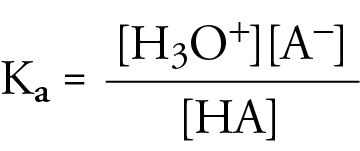
Kb general information:
Kb = [BH+][OH-] / [B]. The products (conjugate acid and conjugate base) are on top, while the parent base is on the bottom. Once again, water is not present.
Strong Acids:
A strong acid (HA) completely dissociates in water, meaning the reaction goes to completion. Therefore, they do not have a significant Ka or Kb value, as there is no equilibrium.
Example: HCl(aq) + H₂O(l) → H₃O⁺(aq) + Cl⁻(aq)
Weak Acids:
A weak acid (HA) only partially dissociates in water, establishing an equilibrium between the undissociated acid and its ions.
Example: HA(aq) + H₂O(l) ⇌ H₃O⁺(aq) + A⁻(aq)
How does the Ka and Kb of strong and weak acids link to the Kc equation?
The Ka equation is obtained by using the Kc equation for the equilibrium reaction of the acid’s ionisation in water, where the reactants are the acid (HA) and water, and the products are those for ionisation of the acid: H3O+ and A-.
Water is not included in the equation, so the Kc equation becomes the Ka equation
What are the steps for solving problems wit Ka, Kb and pH?
It depends on what the question is asking. pH → [H+]
If an initial concentration is given, a RICE table can be used, with the pH being used to find [H+] at equilibrium, and then solve for Ka.
If Ka is given, you can determine the [H+] at equilibrium, assuming the change ‘x’ is small.
By solving for x, (or [H+]), the pH can be found. Kb problems are solved as for Ka problems, but would relate to ionization of a base rather than an acid.
How can percentage ionisation of an acid can be found using pH?
% ionisation = [𝐻3𝑂 +] [𝐻𝐴] x 100.
The pH value can be used to find [𝐻3𝑂 +] by using [𝐻3𝑂 +] = 10−𝑝𝐻.
Why are pKa and pKb used rather than Ka and Kb in some contexts?
pKa formula: pKa = -log (Ka)
pKb formula: pKb = -log (Kb) or pKb = 14 - pKa
pKa of strong acids: Small pKa values. (In the negatives)
pKa of weak acids: Large pKa values.
pKb of strong bases: Small pKb values.
pKb of weak bases: Large pKb values
Practice question:
