pchem exam 2
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
50 Terms
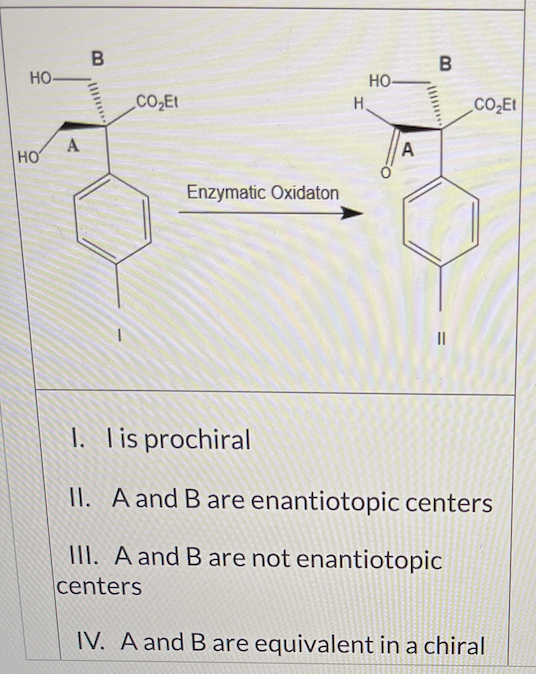
In theory, the diol I can be enzymatically oxidized to the aldehyde Il (see scheme below).
Which of the following statements about I are valid? (there may be more than one that apply)
I and II only
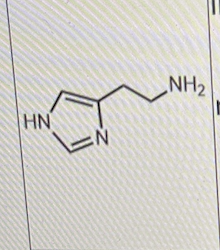
Which of the following is/are correct about the molecule shown below?
I. it is involved in local immune response
II. it is involved in the regulation of physiological function in the gut
III. all of its receptors are GPCRs
I, II, and III
Which of the following enzymes can be involved in the metabolic reaction shown below? - OXIDATIVE DEAMINATION
I. alcohol dehydrogenase
II. CYP450
III. MAO
II and III only

Which of the following is/are correct about the molecule shown below?
I. It can cross the blood brain barrier
Il. It is a substrate of COMT
IIl. It is charge neutral under physiological pH
I, II, and III
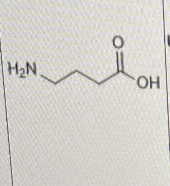
Which of the following is/are correct about the molecule shown below?
I. it is highly water soluble
II. it is charge neutral under physiological pH
III. there is no reuptake mechanism for its elimination from the synapse
I and II only
a molecule that is a 50:50 mix on enantiomers is
racemic
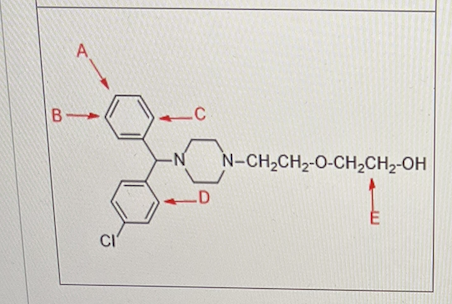
The drug below is know to undergo metabolically aromatic hydroxylation reaction.
Where is the hydroxyl group most likely to go for the aromatic hydroxylation?
A
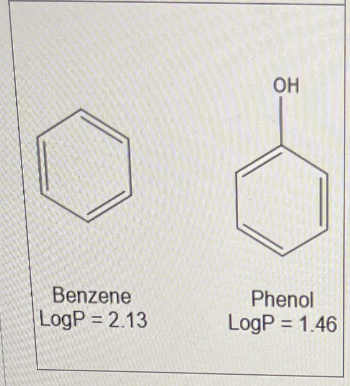
Below are benzene and phenol.
The logP for benzene is 2.13 and the logP for phenol is 1.46
Calculate the πOH Value
-0.67
True or False:
Phase 2 conjugation reactions always lead to increase in water solubility.
False
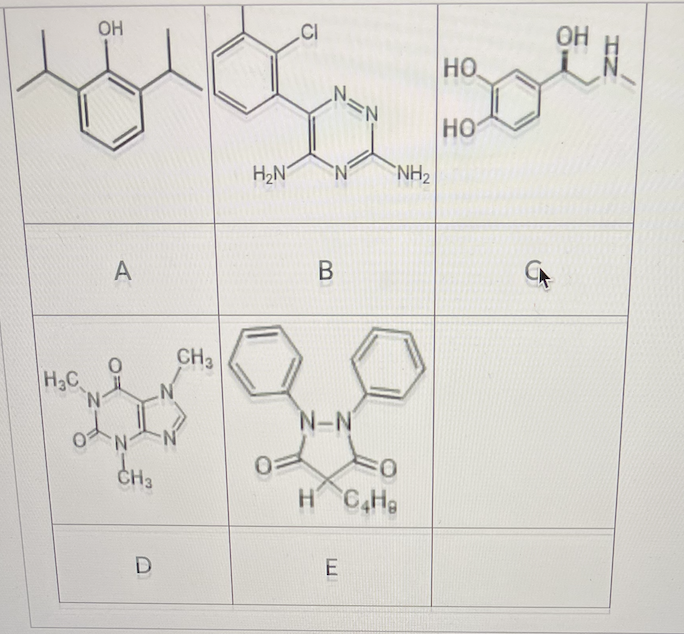
Which of the following compounds can undergo direct glucuronidation?
A, B, C, E
Which of the following receptors can the molecule shown below bind to and activate?
I. AMPA receptor
Il. Kainate receptor
III. NMDA receptor
I, II, and III
What is the intermediate in O-dealkylation?
hemiacetal
Which of the following is/are correct about the molecule shown below?
I. It is the neurotransmitter serotonin
Il. It is eliminated from the synapse through reuptake
IlI. It has a net positive charge under physiological pH
I, II, and III
Which of the following functional groups/compounds can be acetylated metabolically?
amino acids, aromatic amines, hydrazaine, sulfonamides
NOT TERTIARY AMINES
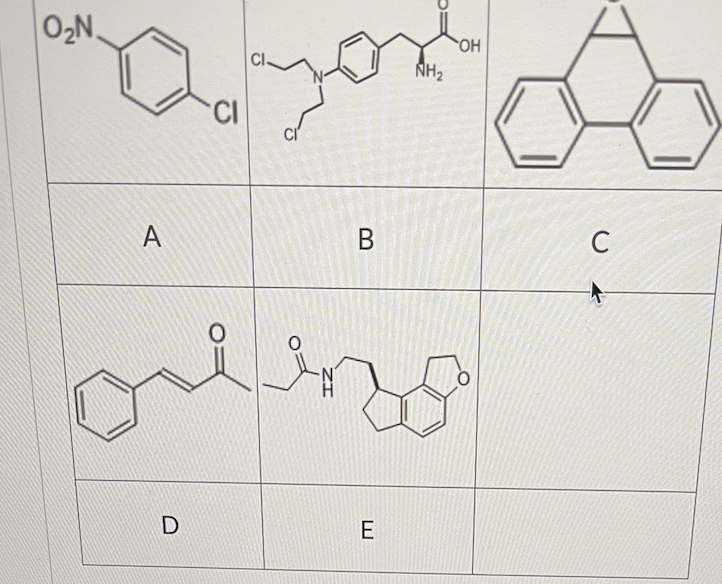
Which of the following compounds/drugs can be a direct substrate of glutathione S-transferase?
A, B, C, D
which of the following functional groups can be reduced metabolically?
aldehyde, aromatic nitro, azo, ketone
complete the sentence to make it true: the lower the πx value,
the lower the lipophilicity
True or False:
An aldehyde is more likely to be oxidized metabolically than reduced.
True
What is the cosubstrate in metabolic acetylation reactions?
CH3CO-SCoA
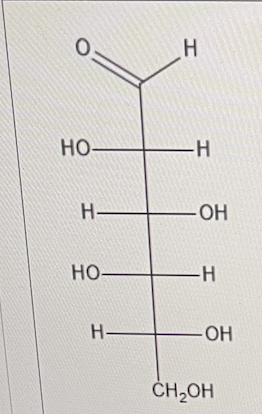
For Idose, use the glyceraldehyde rule to determine if the configuration of Idose is D or L
D
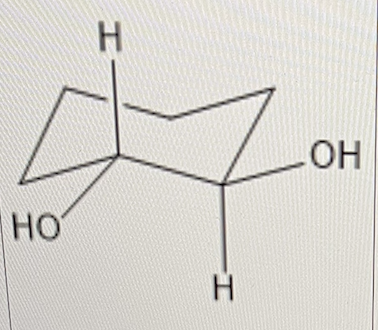
For 1,2-cyclohexane diol, determine if the configuration is cis or trans
trans
True or False:
All cholinergic receptors are ligand-gated ion channels.
False
Which of the following is/are correct about the nerve gas Soman?
I. it inhibits choline acetyltransferase
II. it inihibits the hydrolysis of acetylcholine
III. it increases the amount of acetylcholine in the synaptic cleft
II and III only
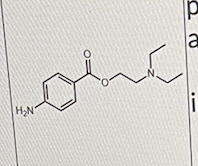
The drug below is used only as a local anesthetic because
I. It is too lipophilic to be absorbed from the injection site
II. It has no other pharmacological activity at sites other than the injection site
III. It is quickly hydrolyzed by esterases once it is absorbed from the injection site
III only
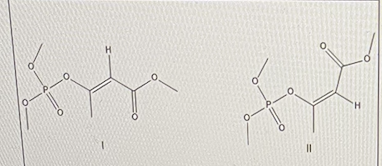
For Mevinphos, determine which isomer is E and which is Z
I = E; II = Z
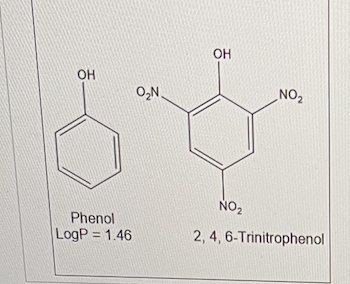
If the logP value of phenol is 1.46 and the πNO2 is -0.28, determine the logP value for 2,4,6-trinitrophenol
0.62
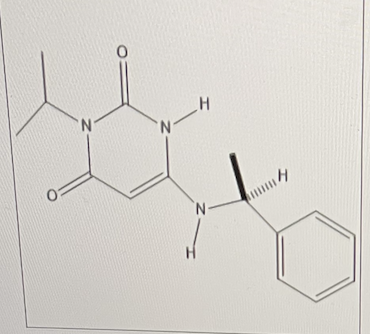
Mavamectin is in Phase III clinical trials for treatment of cardiomyopathy.
Assign the configuration to the chiral center.
S
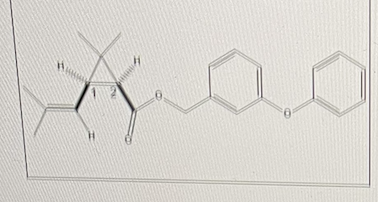
Permethrin can be used as an insecticide and as a drug to treat scabies and lice.
Assign stereochemistry to centers 1 and 2, respectively
R,S
What is the cosubstrate in metabolic sulfation reactions?
PAPS
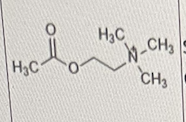
Which of the following is/are correct about the molecule shown below?
I. It is synthesized in the synaptic cleft
II. it is highly water soluble due to the quaternary ammonium cation
III. it can produce excitatory actions
II and III only
True or False:
Hydrolysis of esters is a common phase 1 oxidative metabolic reaction.
false
The combination of Levodopa and carbiDOPA can be used to treat Parkinson's Disease because
I. Parkinson's Disease is related to the deficiency of Levodopa in the brain
Il. carbiDOPA is an inhibitor of the peripheral decarboxylase
III. carbiDOPA helps to extend the plasma half-life of Levodopa
II and III only
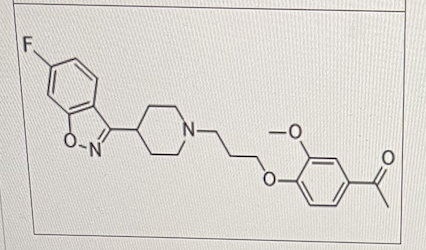
Select All That Apply:
lleperidone is an antipsychotic agent indicated for schizophrenia.
Which of the following metabolic reactions can happen to iloperidone directly?
N-Dealkylation
O-Dealkylation
Reductive reaction
What is the intermediate in N-dealkylation?
carbinolamine
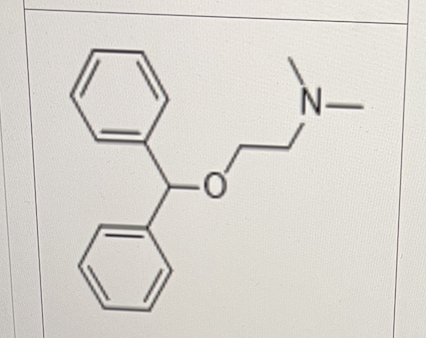
Select All That Apply:
Diphenhydramine (Benadry®) is an antihistamine mainly used to treat allergies.
Which of the following metabolic reactions can happen to diphenhydramine directly?
aromatic hydroxylation
deamination
N-dealkylation
O-dealkylation
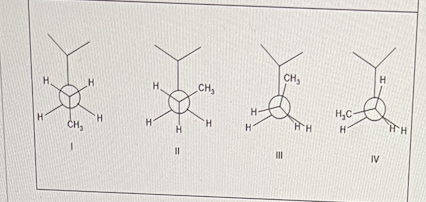
Below are 4 conformers of 2-methyl pentane.
Which conformer has the lowest energy?
I
True or False:
All metabotropic glutamate receptors are GPCRs.
True
What is the intermediate in S-dealkylation?
thiohemiacetal
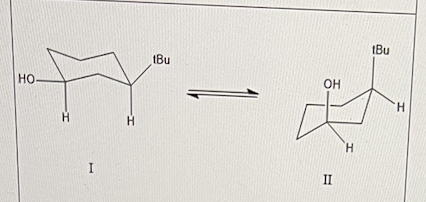
The scheme below shows an equilibrium between two cyclohexane conformations.
Which conformer will have the lowest energy?
I
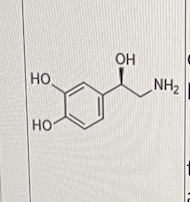
Which of the following is/are correct about the molecule shown below?
I. It is a neurotransmitter present only in the central nervous system
II. It is negatively charged at physiological pH
III. It is eliminated in the synapse through the action of catechol O-methyl transferase (COMT) as one of the mechanisms
III only
Which of the following enzymes can be involved in the metabolic reaction shown below? - N-OXIDE formation
I. aldehyde oxidase
II. CYP450
III. Flavin-containing monooxygenase (FMO)
II and III only
Select All That Apply:
What are the enzymes involved in mercapturic acid formation?
Acetylase
Cysteinyl-glycinase
Glutamyl transferase
Glutathione S-transferase
The concentration of a drug in the lipid phase divided by the concentration
of a drug in the aqueous phase is known as what?
the partition coefficient
True or False:
Drug Metabolism always leads to inactivation of a drug.
False
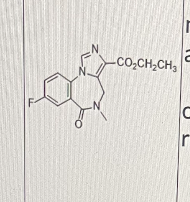
Flumazenil is a benzodiazepine receptor antagonist used to treat benzodiazepine overdose.
It has a short half-life of 7 - 15 min after iv administration.
What is/are the most likely reason(s) that its half-life is so short?
I. quick elimination through glucuronidation
II. quick metabolism through aromatic hydroxylation on the left benzene ring
III. quick hydrolysis of the ester bond by serum esterases
III only
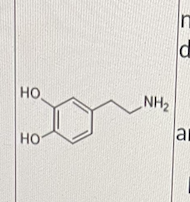
Which of the following is/are correct about the molecule shown below?
I. It is the neurotransmitter dopamine
II. All of its receptors are GPCRs
III. It has a net positive charge under physiologica pH
I, II, and III
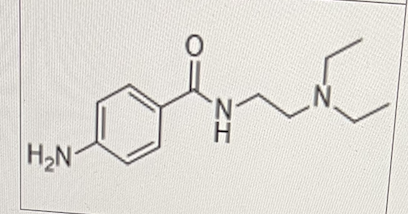
select All That Apply:
Which of the following metabolic reactions can happen to the drug below?
N-dealkylation
Deamination
Hydrolysis of the amide bond
True or False:
Drug metabolism can happen in the kidneys.
True
Which of the following compounds can be a substrate of UDP-glucuronyl transferase?
Alcohols
Amines
R-SH (sulfhydryl)
Carboxylic acids
Phenols

Terfenadine (Seldane®), a second generation antihistamine, was converted in vivo extensively to fexofenadine. Fexofenadine was later developed as a third generation antihistamine - Allegra®.
What is/are involved in the metabolic conversion from terfenadine to fexofenadine?
I. aliphatic hydroxylation
II. oxidation of alcohol to aldehyde
III. oxidation of aldehyde to carboxylic acid
I, II, and III