1.2B Isotopes
1/5
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
What are Isotopes?
atoms of an element that have the same number of protons but a different number of neutrons.
What are the qualities of different isotopes?
similar chemical properties
different atomic masses
different physical properties
Do isotopes have similar chemical properties? Why or why not?
they do have similar chemical properties because the number of protons and electrons are the same
How does atomic mass relate to their separation methods?
Heavier isotopes move more slowly at a given temperature and these differences can be used to separate isotopes.
What is the equation for finding Relative Atomic Mass?
Relative Average Mass = (% abundance of isotope 1)(mass of isotope1) + (% isotope2)(mass isotope2) +…
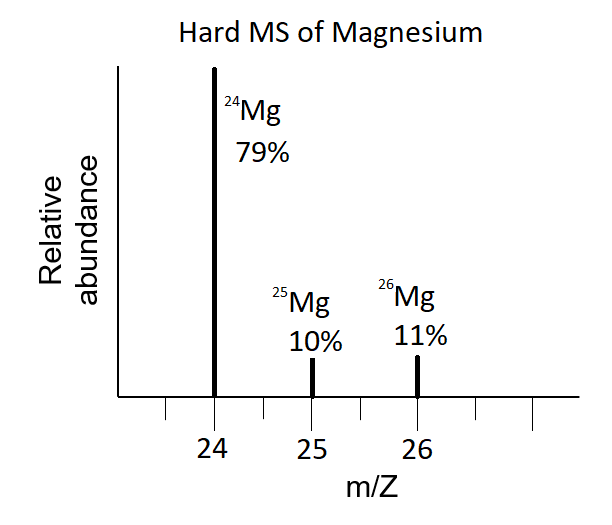
Tell me about Mass Spectra
An instrument known as a mass spectrometer can be used to measure the mass of individual atoms. The results are presented as a mass spectrum, where the percent abundance is plotted against the mass/charge ratio of different ions. The relative average mass can be calculated from this data.