Molecules of Life
1/248
Earn XP
Description and Tags
Block 1- Amino Acids and Proteins, Block 2- Enzymes and Bioenergetics, Block 3- Carbohydrates and Lipids, Block 4- Animal Metabolism
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
249 Terms
Number of core amino acids
20
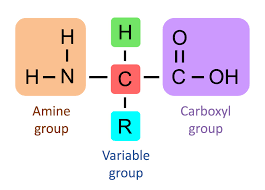
Structure of an amino acid
What is chirality
Molecules which are mirror images of each other and cannot be superimposed. These molecules are known as enantiomers.
What is a chiral isomer
a carbon with 4 different groups attached
How do you tell the difference between an L and D isomer
L- isomer: the groups spell CORN clockwise
D-isomer: the groups spell CORN anticlockwise.
Which isomer are proteins made of and why
Exclusively L isomers. Because the biochemical reactions are stereospecific
Why is glycine non-chiral
Because its R group is a hydrogen therefore not all the groups are different and chirality is not achieved.
What are the intermolecular forces in order of strength
Covalent - Salt bridge - Hydrogen - Van der waals
How are covalent bonds formed in a protein
Disulphide bridges. They are formed by cysteine (which has a sulphur in its R group) forming a disulphide bridge with a neighbouring cysteine. This happens through an oxidation reaction.
How are salt bridges formed in a protein
They are electrostatic attractions between amino acids with R groups with opposing charges. Opposites attract, same repel.
How do Van der waals forces operate in a protein
They are distance dependent and weak. They depend on free moving electrons transiently locating to end of a molecule, making one side partially negative and one side partially positive.
How do hydrogen bonds operate in a protein
Electronegative elements (Nitrogen, Oxygen, Flourine) pull electrons towards them when in a bond with hydrogen. This creates a permanent dipole with hydrogen partially positive and the electronegative element partially negative.
How does the hydrophobic effect work in proteins
Non-polar hydrophobic regions cluster together to present the smallest hydrophobic area to the surrounding water. The surrounding water will then hydrogen bond around the protein, forming a “cage”
What does amphipathic mean
A compound which contains both polar and non polar regions.
What are zwitterions and how are they formed.
At pH 7.4 the amino group will be positive and the carboxyl group will be negative. This means it has both negative and positive ends, making it a zwitterion.
Which amino acids are hydrophobic
Glycine (G), Alanine (A), Proline (P), Valine (V), Leucine (L), Isoleucine (I), Methionine (M)
Which amino acids are aromatic hydrophobic
Phenylalanine (F), Tyrosine (Y), Tryptophan (W)
Which amino acids are hydrophillic/polar
Serine (S), Threonine (T), Cysteine (C), Asparagine (N), Glutamine (Q)
Which amino acids are positively charged
Lysine (K), Arginine (R), Histidine (H)
Which amino acids are negatively charged
Aspartate (D), Glutamate (E)
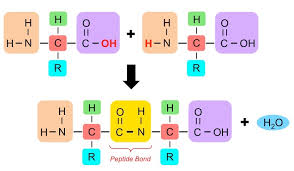
How are peptide bonds formed
Through a condensation reaction which produces water
How are alpha helices formed
Hydrogen bonding between C=O and N-H 4 amino acids away from each other. Almost all amino acids in the chain will form this bond. The R chains orient away from the centre.
Which amino acids are not found in an alpha helix
Glycine and Alanine - Not found because their R group is too small and flexible
Proline- Not flexible enough.
How is protein purification conducted
It uses the biochemical properties (Size, charge, hydrophobicity. solubility) to purify it from a mixed solution.
What are protein motifs
Secondary structural elements which can link alpha helices and bets sheets.
How are turns formed
Consist of 4 AAs. Uses glycine for flexibility or proline for a tight bend
How are disordered loops formed
Rich in polar, uncharged AAs. This allows larger flexibility and degree of rotation.
What are the 4 classes of proteins
Fibrous
Globular
Membrane
Intrinsically disordered
What are fibrous proteins
Contain simple, repeating structural elements, contains only 1 type of secondary structure. They are hydrophobic and water insoluble.
Eg. Collagen, a-Keratin, Fibroin
What are globular proteins
They are structurally diverse and compact. They usually have a dense hydrophobic core with hydrophilic residues on the surface.
Eg. Enzymes and transport proteins
What are membrane proteins
Embedded in the phospholipid bilayer. Internal faces of transport proteins are exposed to the aqueous environment. Gram -ve bacteria, mitochondria and chloroplasts have a b-Barrell structure with a polar internal face.
What are intrinsically disordered proteins
They are used as signalling hubs, can be entire proteins or discrete regions
How are quaternary structures formed
Individual polypeptide chains forming a protein complex
How are quaternary structures named
Hetero/Homo, if the subunits are different/same
Number of subunits (eg. 4 = tetra)
Mer / meric
eg. Heteropentameric
What are prosthetic groups
They are permanently associated with a protein. Eg. Haem group in haemoglobin
What are co-enzymes
An organic or metallorganic which is transiently bound to an organic cofactor, essential for active site function. Eg. acetyl co-enzyme A
What are metals as a cofactor
An inorganic metal ion Eg. Zinc
What are the types of protein modifications and which amino acid do they affect

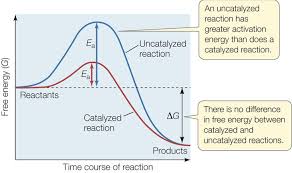
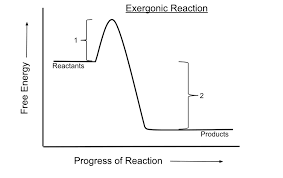
Show a catalysed vs uncatalysed exergonic reaction
What is potential energy
Hidden energy as a consequence of position (stored energy)
What is kinetic energy
Energy as a consequence of motion
Why do chemical bonds have potential energy
As a consequence of bonds between atoms
What is Gibbs free energy
The amount of useful energy released / needed in a reaction
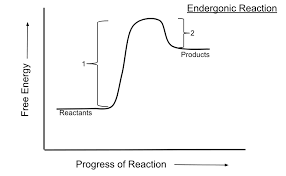
What are the features of an endergonic reaction
Products have more potential energy than reactants
Gibbs is greater than 0 so reaction is unfavourable
What are the features of an exergonic reaction
Reactants have more potential energy than products.
Gibbs is less than 0, so reaction is favourable
What is the definition of rate of reaction
The change in the amount of reactants/products over time
What makes a successful reaction collision
Energy greater than the activation energy, correct orientation
How does temperature affect rate of reaction
Higher temp = higher kinetic energy = increased particle movement = more successful collisions
How does reactant concentration affect rate of reaction
More molecules in the same volume = more collisions
How does use of a catalyst affect rate of reaction
it decreases the activation energy by holding reactants in an orientation which reduces Ea. It does this by using covalent and non covalent interactions in the enzyme-substrate complex position.
What is a cofactor
1 or more inorganic ions which can stabilise charges in the active site or accept/donate electrons.
What is a holoenzyme
A completely catalytically active enzyme with bound cofactors and / or co enzymes
What is an apoenzyme
The protein part of an enzyme
What are the 5 different types of enzyme
Oxioreductases- Moves electrons/hydrogen ions from one molecule to another
Transferases- Moves a chemical group from one molecule to another
Hydrolases- Breaks down a molecule using water
Isomerases- Re-arranging groups within the substrate molecule (creates an isomer)
Ligases- Joins molecules together (ligation)
What is the difference between catabolism and anabolism
Catabolism is breaking down molecules and it generates energy, anabolism is building up and it requires energy.
What is the induced fit model
An enzyme is flexible and changes shape, this forms the active site when it encounters the substrate
The enzyme will undergo a conformational change when the substrate binds
This provides the driving force for the reaction by stabilising the transition state and lowering the activation energy
What is Vmax
The maximum reaction velocity of an enzyme catalysed reaction, when an enzyme is saturated at a defined enzyme concentration
What is Km
The substrate concentration at ½ Vmax. It can be used as a measure of substrate concentration needed for an enzyme to function at ½ Vmax (optimal)
What does a large Km indicate
Weak binding between the enzyme and substrate (low affinity)
What does a small Km indicate
Strong binding between enzyme and substrate (high affinity)
What is the active site
A 3D cleft containing catalytic amino acids
Which structural biology techniques are used to understand enzyme mechanisms
X ray chrystallography
NMR
Electron microscopy
What are the general features of proteases
They are hydrolase enzymes which can cleave peptide bonds
What are the functions of serine proteases
When serine residues are found in the active site of a protease
They function in
Digestion
Blood clotting
Fertilisation
Immunity
Serine protease needs to be activated by a different serine protease
What is chemotrypsin
A serine protease which is used for peptide bonds with a aromatic amino acid adjacent. They use a hydrophobic pocket close to the active site to position the substrate
How does the catalytic activity in chemotrypsin occur
Serine acts as a nucleophile and histidine acts as a base
Uses the catalytic triad of Ser, His and Asp which forms a H bond network
The residues bond to the substrate to form an intermediate
This breaks the peptide bond
The residues are regenerated
The active site also contains an oxyanion hole close to Gly 193 which stabilises negative charge on the intermediate
Explain the HIV lifecycle
It is a retrovirus. It replicates using 3 enzymes.
Reverse transcriptase converts viral RNA to DNA
Integrase inserts the viral DNA into the host chromosome
Protease produces new viral proteins
The specific protease is aspartyl protease which cleaves bonds betwen phe +pro, and uses a hydrophobic pocket
How are researchers producing antiretroviral medication for HIV
Using inhibitors with aromatic groups to inhibit the aspartly protease used in retroviral replication
What happens to a reaction when physiological concentrations are greater than Km
Fast rate
Low sensitivity to changes in substrate concentrations
What happens to a reaction when physiological concentrations are less than Km
Slow rate
High sensitivity to changes in substrate concentrations
What is Kcat / turnover number
It is independent of enzyme concentration, it describes the number of substrate molecules converted to product by a single enzyme in a given unit of time
What does the ratio of KM/Kcat tell us
Enzyme efficiency. Accounts for rate of catalysis for a substrate (Kcat) and the affinity of the enzyme for the substrate (KM)
What are the types of reversible binding which regulate enzymes
Non-covalent binding of enzyme modulators (allosteric effectors), usually small metabolites or cofactors
Covalent modifications - addition of a phosphoryl group
Binding of regulatory proteins
What is competitive enzyme inhibition
Molecules similar to the substrate (such as the reaction product) can bind to the active site, inhibiting the reaction
What is non- competitive enzyme inhibition
Inhibitor binds at a site distinct from the active site (allosteric site) and bring about a conformational change in the enzyme, thus inhibiting or activating the enzyme
What are allosteric effectors
Inhibitors and enhancers
Effectors bind to sites distinct from the active site, often within different regulatory subunits than catalytic subunits
The conformational change interconverts an enzyme from a relatively inactive ‘tense’ T state to a more active ‘relaxed’ R state
How does competitive inhibition affect enzyme activity
Vmax remains the same as high substrate concentration can outcompete the inhibitor
Km increases as more substrate is needed to compete with inhibitor at ½ Vmax
How does non- competitive inhibition affect enzyme activity
Vmax decreases as conformational change reduces enzyme activity
Km remains the same as there is no competition at the active site.
How does negative feedback regulation control ATCase
ATCase is the first step in pyrimidine biosynetthesis. Its activity is regulated by pathway product, CTP, in order to confirm the right amount of CTP is made in the cell. If enough CTP is present, it will allosterically inhibit ATCase to ensure the correct amount is made.
What is cooperative behaviour
When the binding of a substrate to one site increases the affinity of substrate binding to the other sites of an enzyme
What are some advantages of using enzymes in biotechnology
Easy to manipulate biological systems to alter their properties eg. express genes of interest and mutagenise DNA to change coding sequence to alter properties
Natural biodiversity eg. wide variety of naturally occurring sources of enzyme, good reproducibility and high catalytic activity.
What is regiospecificity
Enzymes bind and recognize substrates in such a way that they can selectively transform a complex substrate at a specific site . Important for production of pure materials.
What is stereospecificity
Many biomolecules exist as enantiomers, therefore it is important in biotech production to produce only one specific stereoisomer
What are some disadvantages of using enzymes in biotechnology
They have a limited temperature range for which they are active
They also have a limited pH range
How does allosteric binding not change the tertiary or secondary structure to inhibit binding
Inhibitors will bind to regulatory subunits rather then catalytic subunits in an enzyme. This induces a conformational change in the quaternary structure rather then disrupting the 3D structure of the subunits. The conformational change will then affect the active site structure which will reduce its activity.
What are some functions of carbohydrates
Fuel
Energy storage
Metabolic intermediates
Structural backbone of DNA and RNA
Cell walls
What is the base formula of a monosaccharide
CnH2nOn
How are monosaccharides named
The number of carbon atoms eg.
C3H6O3 is a triose
C4H8O4 is a tetrose
C5H10O5 is a pentose
C6H12O6 is a hexose
What is the difference between an aldose and a ketone
If the C=O is on the terminal end of the monosaccharide it is a aldose
If the C=O is inside the monosaccharide then it is a ketone
Can aldehydes and ketones be chiral
Yes they can contain chiral carbons, aldehydes more then ketones
Which L/D monosaccharide isomer is more prevalent in living organisms
D
How is a monosaccharide deemed L or D
If the hydroxyl group on the second to last carbon is Left = L isomer
If the hydroxyl group on the second to last carbon is right = D isomer
What is an epimer
Structure only differs by stereochemistry at only one carbon chiral centre, formula is the same
How is the number of possible monosaccharide structures determined
2 to the power of the number of chiral centres
How do monosaccharides form rings
Ring formation through generation of a covalent bond between C1 and the hydroxyl group from the second from last carbon
What is an anomer
Alpha or beta form, where the carbon points up or down
What are some examples of monosaccharide modifications
Loss of an oxygen atom determines DNA or RNA and leads to the nucleic acid having a very different role in the cell
Addition of amine groups important for biosynthesis of glycosylated proteins and lipids
Phosphorylated sugars are important intermediates in metabolic pathways
What do fatty acids contain
Carboxylic acid groups with a hydrocarbon (C and H) chain
Chains are 4-36 carbons in length
What is the difference between saturated and unsaturated fatty acids
Saturated (no double bonds) or unsaturated (one or more C=C bonds)