1. Carbohydrates
1/114
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
115 Terms
Monosaccharides
E.g. glucose & fructose
Oligosaccharides
2-10 monomers
Disaccharides (glucose + fructose)
Trisaccharides (galactose + glucose + fructose)
Polysaccharides
>10 monomers
Starch: 1000-2×10^6 glucose monomers
Cellulose: 7000-15000 glucose monomers
Pectin: 200-500 monomers
Isolation to form pure ingredients
Sugar made from sugar cane or sugar beets
Pectin powder made from orange peels
Lactose powder made from milk
Starch powder made from potatoes
Nutritional functionality of carbohydrates
Energy supplier
Fibers
Technical functionality of carbohydrates
Sweetener
Humectant/water binder
Texture: thickener, gelling agent
pre-cursor for color and flavor
Ring opening
Mostly closed (>99%)
But can open (<1%)
Aldose vs Ketose
Aldose =O on C1 (aldehyde)
Ketose =O on C2 (ketone)
Anomeric carbon
C- atom next to O atom in the ring and connected to a second O atom
In an open structure, this carbon is the C-atom connected to the =O
Pyranose vs furanose
Pyranose: 6 membered ring
Furanose: 5 membered ring
Hexose vs Pentose
Hexose: 6 C atoms (glucose & fructose)
Pentose: 5 C atoms (Xylose & arabinose)
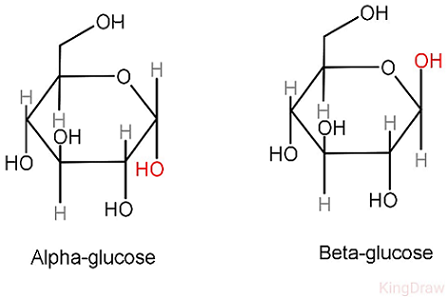
Alpha or beta glucose
Alpha is in starch
Beta in cellulose
D or L
Are mirror images of each other
If the functional group on second last C-atom is to the right then it is D-form
If the functional group on second last C-atom is to the left then it is L-form
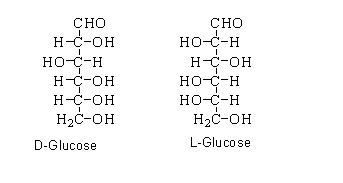
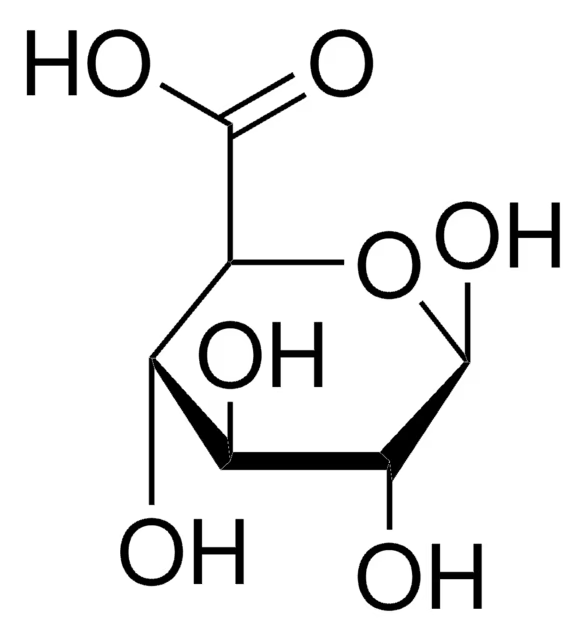
Uronic acids
Has an acid group on the sixth carbon
Instead of a hydroxyl group, it has an acid group
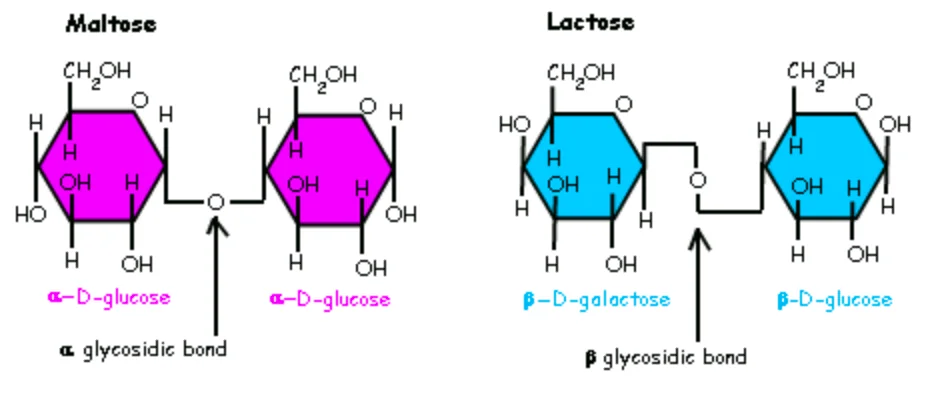
Alpha or beta linkage
Alpha when the linkage is drawn down, beta when zigzagged
If both are up or both are down = alpha
If opposite = beta
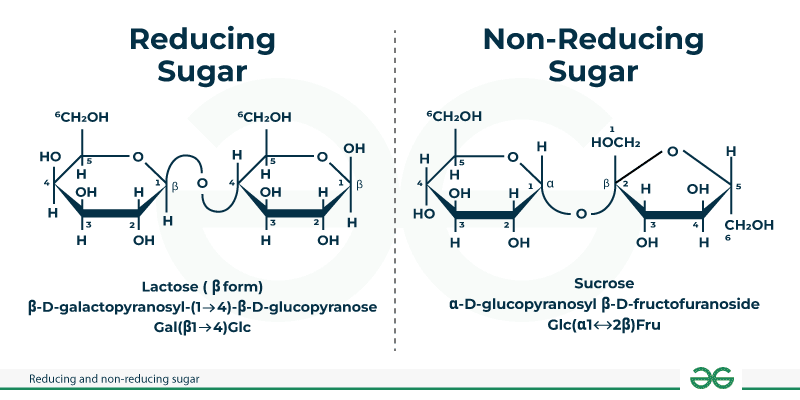
Properties reducing sugar
Can undergo reactions which normal sugars cannot (caramelization, maillard)
They can open up their ring
It can reduce other reactants
OH group is free on the anomeric carbon (not bonded to another molecule)
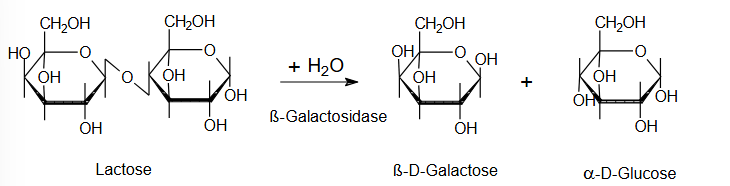
e.g. lactose = reducing
e.g. saccharose = not reducing, has no reducing ends
When it hydrolyses, it can react in a reducing reaction
Calculating Dextrose equivalent
DE = 100 * number of reducing ends/glucose monomers
The more you cut the higher the DE
N and O glycosidic bonds
When an anomeric carbon is bonded to an oxygen or a nitrogen
This means they are not monosaccharides
Mutarotation
When alpha molecules turn into beta molecules and vice versa
Two main groups of polysaccharides
Homoglycan (1 type of monomer)
Heteroglycans (2 or more types of monomer)
Both homo and heteroglycans can exist of linear and branched structures
Example of linear homoglycan polysaccharides
Amylose (alpha 1→ 4 linkages)
Forms a helical structure
More flexible and soluble
Digestible for humans
Cellulose (Beta 1→ 4 linkages)
Very rigid and water in-soluble complexes
Non digestible
Formation of microfibrils
Example of branched homoglycan
Amylopectin
Starch molecule
Consists of D-glucose molecules only
Linkages in the linear part are alpha 1→ 4
Linkages in the branched part are alpha 1→ 6
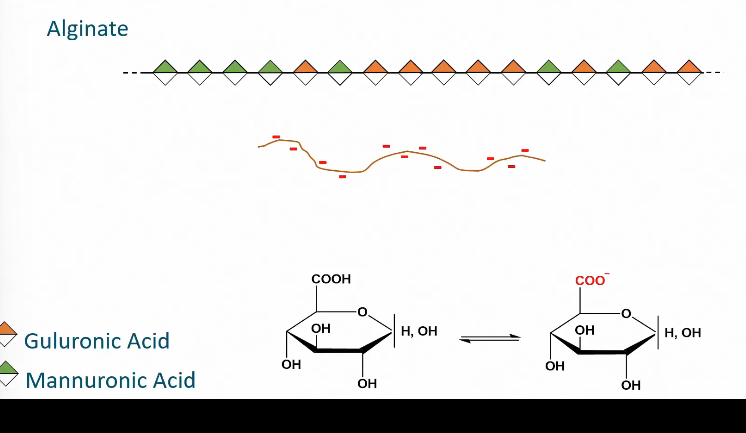
Example of linear heteroglycan
Alginate
Consists of guluronic acid and mannuronic acid
Guluronic acid are monosaccharides with a carboxylic acid group.
Depending on the pH guluronic acid can lose an H on it carboxyl group and become charged
Example of branched heteroglycan
Galactomannan
Consists of galactose and mannose
Techno functionality of polysaccharides
Thickener
Gelling agent
How are polysaccharides responsible for viscosity?
High viscosity = high friction
When there are more polysaccharide chains, friction develops between them, making the solution thick
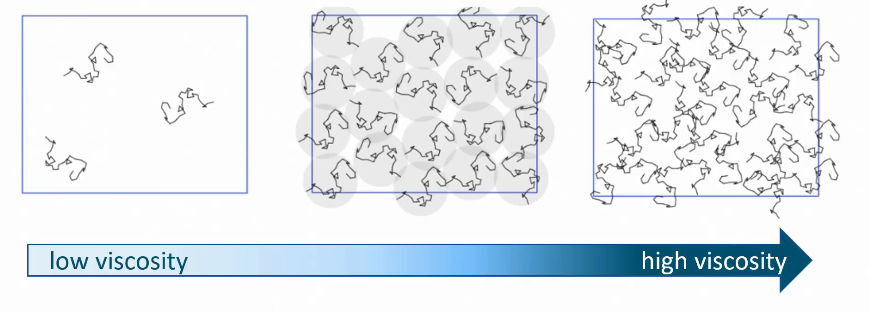
Effect of size, charge & flexibility on thickening
The more space it takes up = the greater the thickening effect.
Larger size = more thickening
More rigid = more thickening
More charge = more thickening
Gelling with polysaccharides
Happens when polysaccharides from junction zones
exist of hydrogen bonds, hydrophobic interactions, electrostatic interactions
They form a 3D network which can hold a liquid.
What are the two roles that polysaccharides usually have in fruits and vegetables?
Storage polysaccharide
Cell wall polysaccharide
Examples of storage and plant cell wall polysaccharides
Storage: starch & glycogen
Cell wall: agar, cellulose and pectin
Properties of sugars
Are hydrophilic
Because of: polar hydroxyl groups (attracts polar water molecules)
Means sugar dissolves well
Limitations to solubility of sugar
If solubility is exceed, the solution becomes over-saturated and crystallization may occur
This means that solid sugar particles are formed in the form of crystals (ordered structures)
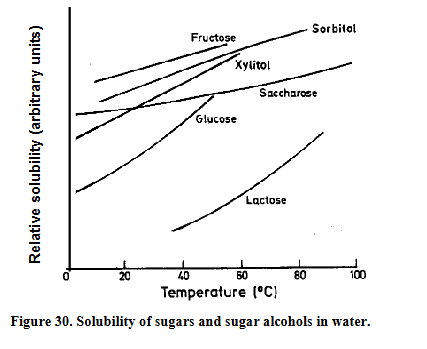
Temperature and solubility
Lactose is the least soluble of the common sugars and fructose has the highest solubility
A big advantage of the use of saccharose is that its solubility hardly depends on the temperature
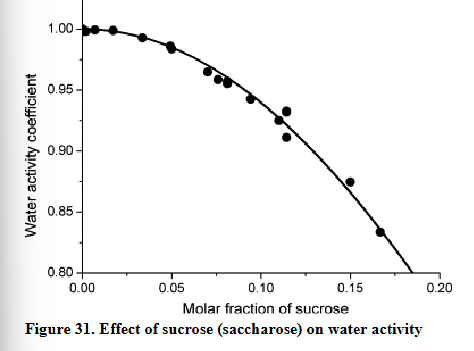
Why do products with a lot of sugar have a low water activity?
There is less free water because the water molecules are bound to the sugar molecule
As a result, water is less free or looses the possibility of free motion
Advantages and disadvantages of low Aw due to high sugar
Advantages: low microbes, slows down reactions that are catalyzed by enzymes and non-enzymatic browning
Disadvantages: The autoxidation of fat is accelerated for foods with aw values below 0.2
Humectants
One of the measures that can be taken to lower the water activity of foods is the addition of additives with a high water binding capacity, these are called humectants
E.g. invert sugar, starch syrups and sorbitol
Fructose can also serve as humectant: the water binding capacity of fructose is larger than that of glucose and saccharose. Therefore, fruit powder is more hygroscopic [hygroscopy is ability to attract and hold water] when the fructose content of the processed fruit is higher (more sugar inversion)
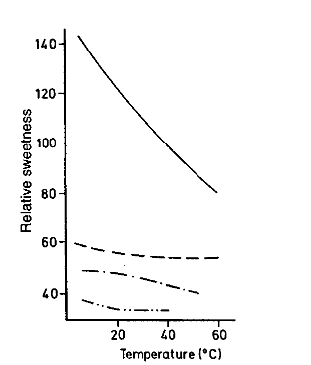
Temperature and sweetness in sugars
Different isomers of fructose vary in sweetness. β-D-fructopyranose is the sweetest form.
As temperature increases, the proportion of β-D-fructopyranose decreases, while the amounts of β-D-fructofuranose and α-D-fructofuranose increase—these forms are less sweet.
Therefore: Fructose tastes sweeter in cold beverages than in hot ones because the sweetest isomer is more abundant at lower temperatures.
NDO’s
Non-digestible oligosaccharides
Are not degraded by the digestive enzymes of the gastrointestinal tract
Therefore they arrive in the large intestine undigested
This way they can play a role as prebiotic
Prebiotic
Prebiotics are defined as compounds that cannot be digested by the human digestive tract
Therefore, they reach the large intestine unchanged
They can stimulate the growth and/or activity of a specific set of health promoting bacteria
Therefore some NDOs are used as prebiotics
Dietary fibre
Includes all soluble and insoluble polysaccharides in a food, with the exception of starch
They are all non-digestible polymers
They do not have a function as energy source
Health effect of dietary fibres
Offer protection against a number of diseases of civilization such as:
atherosclerosis
Constipation
Diabetes
Gallstones
Crohn’s disease
Obesity
Gums
Are added to foods as a fibre additive
Used in breakfast drinks, cereal bars, cookies, cakes, breads etc
Isomerization of monosaccharides via enolization
Known as the “Lobry de Bruyn van Ekenstein isomerization”
Happens at a pH of below 3 or above 7.
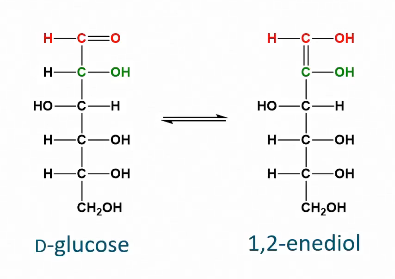
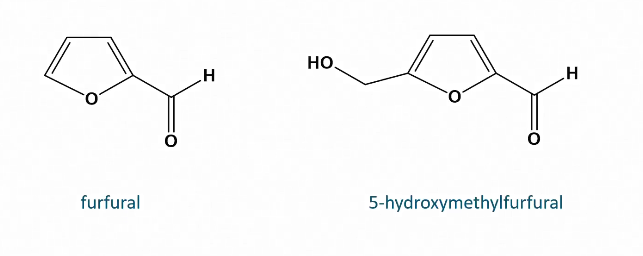
Dehydration of enediol
Forms dicarbonyl
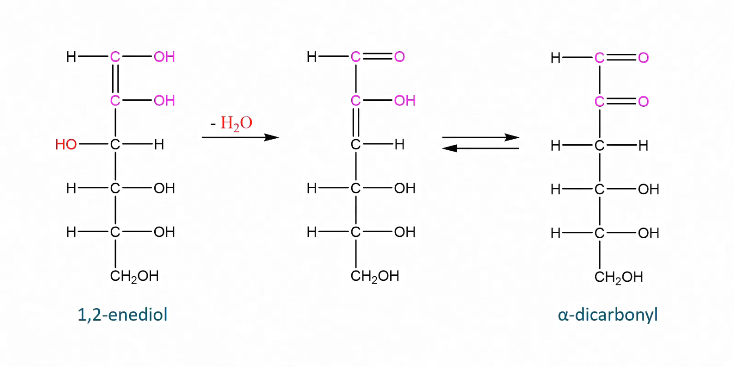
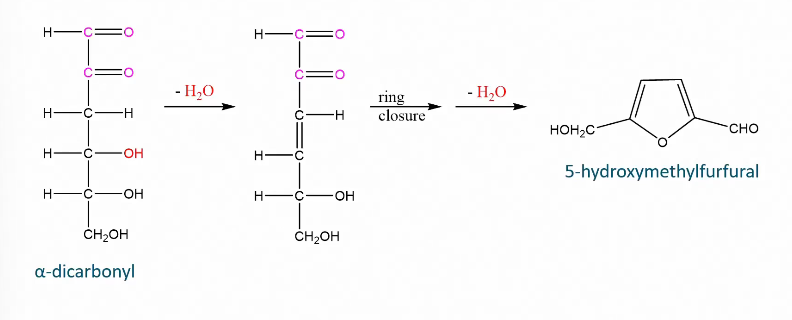
Dehydration of dicarbonyl
Formation of HMF
Higher temp and longer treatment = more HMF
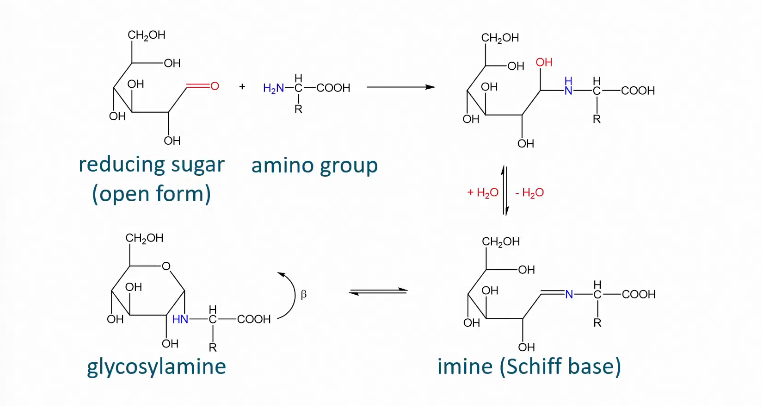
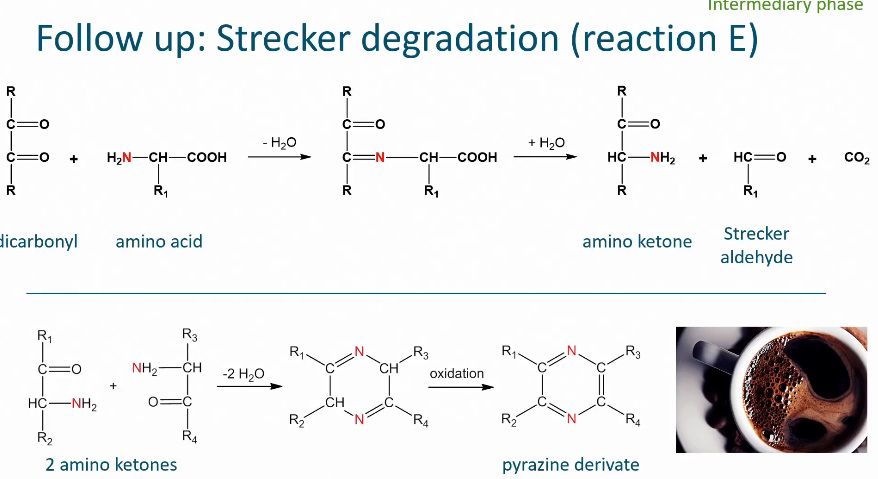
Maillard reactions
When heating carbohydrates and free amino groups Maillard happens
Steps of Maillard reaction
There is no general structure for melanoidins
They are the brown pigments that you see
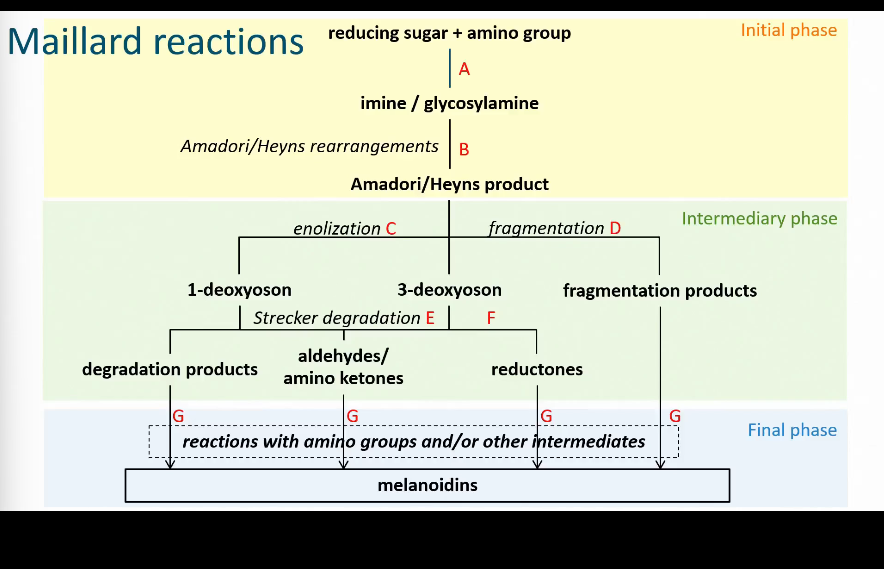
Formation of imine/glycosylamine
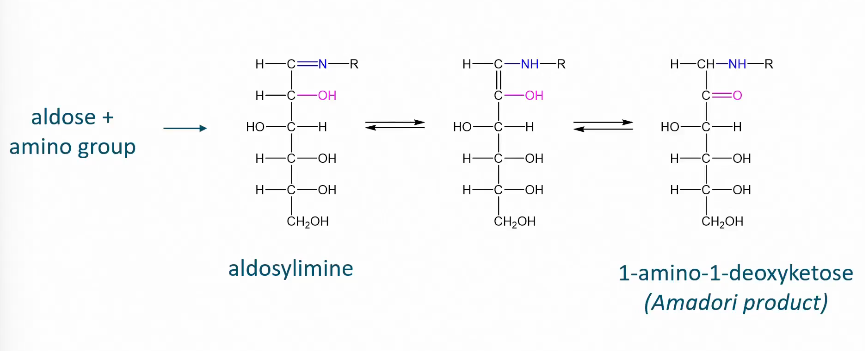
Amadori rearrangement
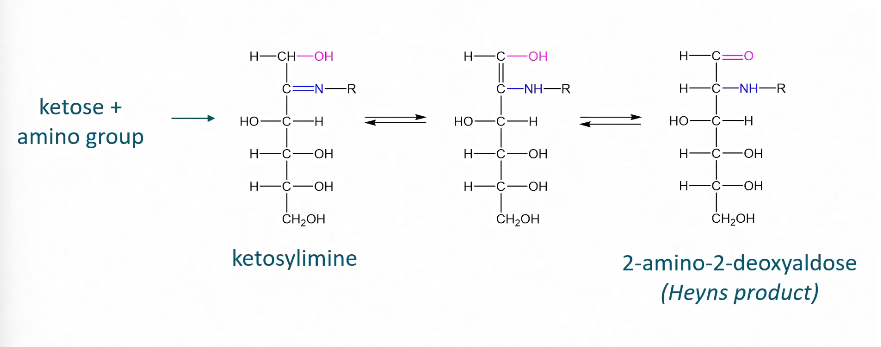
Heyns rearrangement
Enolization Maillard reaction
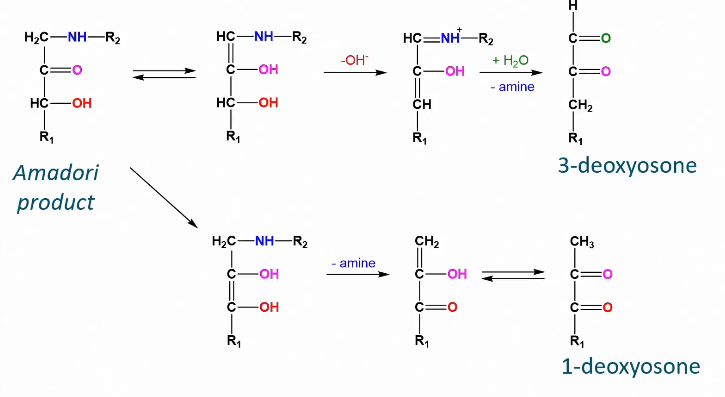
Cyclic intermediate Maillard Reaction
The deoxyosones react further into heterocyclic intermediate products.
Strecker degradation
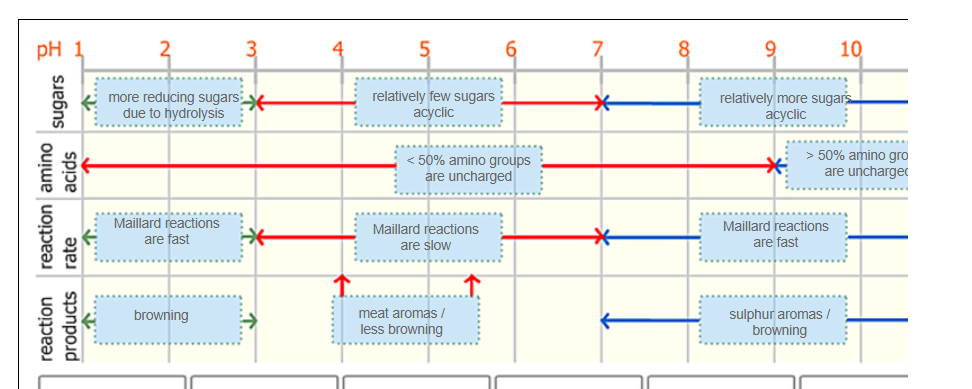
Conditions influencing Maillard reactions
pH
low pH: hydrolysis into reducing sugars
High pH: amino group is in NH2 form
Temperature
Low T (~60C): only initial phase
High T: Also intermediary and final phase
Water activity (balance is optimum)
Low aw: no diffusion of compounds
High aw: no dehydration reactions
Advantages of Maillard reaction
Melanoidins are formed (brown pigments)
Volatile compounds are formed (flavor compounds)
Disadvantages of Maillard reaction
Essential amino acids are lost (lysine, methionine)
Mutagenic or carcinogenic compounds are formed (acrylamide, HMF)
Cross linking of proteins (Loss of functionality/digestibility)
What does viscosity depend on?
Degree of polymerization
Volume
Form
Flexibility
Charge
More space it takes up = larger viscosity
Branched polysaccharides take up less space if they have equal DP as a linear polysaccharide.
Why does a drink with aspartame have less kcal than a drink with saccharose?
Because aspartame has such a high sweetness level that it doesn’t require a lot of aspartame to be added.
Steps in the formation of a gel
Macromolecules turn and fold
Polysaccharides associate
Loops/junction-zones/double helixes are formed
When there are enough knots, a 3D network will arise
Water is retained in this network: a gel
When does the Maillard reaction happen?
When:
Sugars are acyclic
There are a lot of NH2 instead of NH3+ groups in amino acids/peptides
Effect of pH on maillard
Low pH = lower reaction rate, however the amount of Maillard reactions is higher as hydrolysis takes place at a low pH
Hydrolysis produces the substrates for the Maillard reactions, such as reducing sugars and amino acids)
Effect of sulphite on browning?
Sulphite inhibits browning because it reacts with carbonyl intermediates (blocking formation of colored melanoidins).
are pentoses or hexoses more reactive when browning?
Pentoses
Structure of pectin
65% homogalacturonan (important for its gelling properties)
35% rhamnogalacturonan I and II (highly branched)
Structure of homogalacturonan part of pectin
Consists of D-galacturonic acid
alpha 1→ 4 linkages
DP of 60-100
Degree of methyl esterification (DM)
Varies between 20% - 80%
Low DM = LM pectin, meaning that DM < 50%
High DM = HM pectin, meaning that DM = 60-75%
How are junction zones stimulated in a sugar acid gel
Formation of junction zones is stimulated by:
Interaction of two pectin polymer chains
Low pH - to ensure no charge on the pectin so the chains will not repel each other
High concentration of sugar - gives low Aw so that it draws away water from pectin molecules, which makes the pectin molecules less soluble causing them to come closer and form hydrogen bonds & hydrophobic interactions with each other
Typically and HM pectin used for this type of gel, because there are less carboxyl groups that could carry a charge and the methyl groups make the pectin more hydrophobic
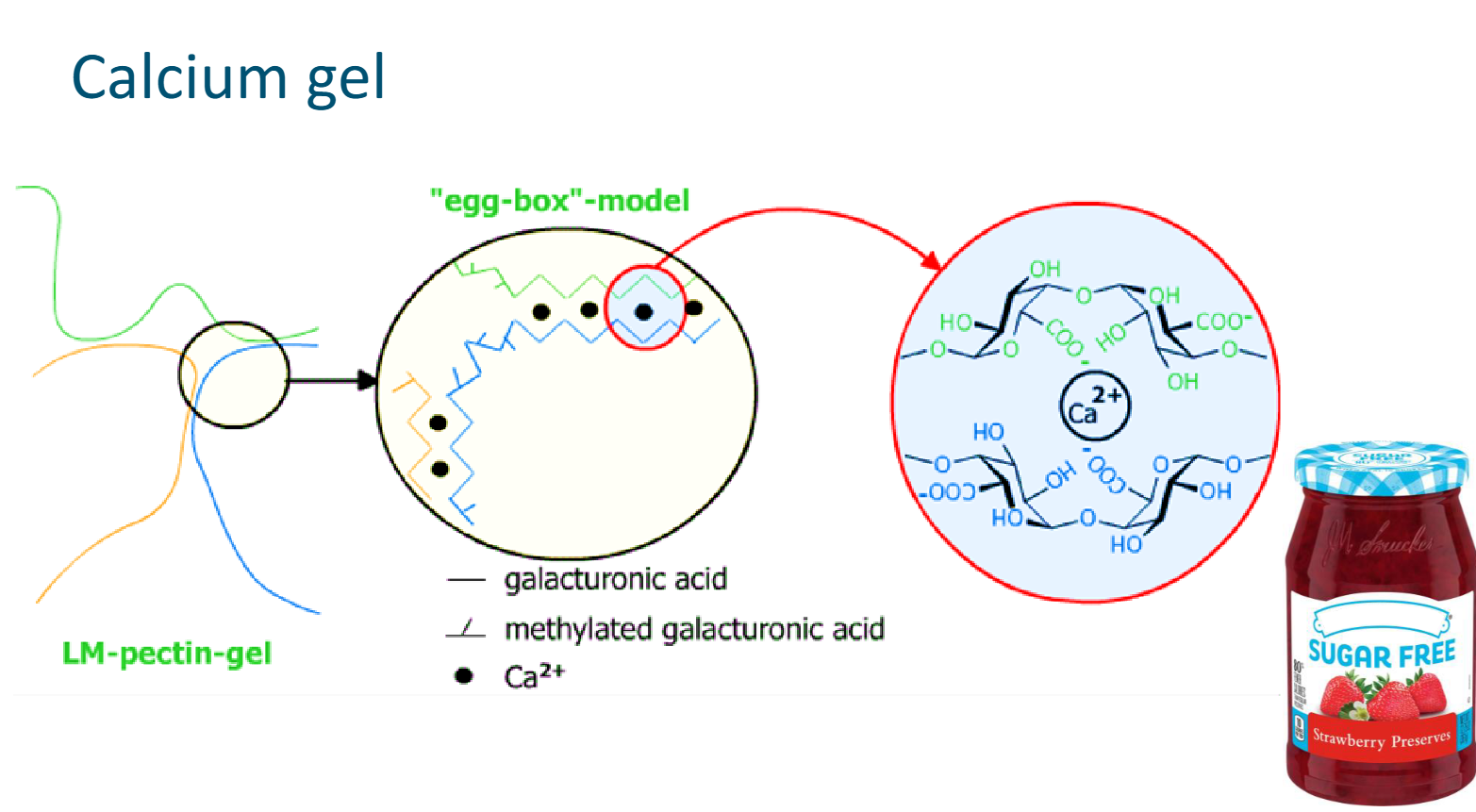
Calcium gel
The negatively charged groups on the pectin form ionic bonds with positively charged calcium ions, forming a so-called eggbox model
The eggs are the calcium ions that are trapped between two polymers
Several calcium ion bonds in a row form a junction zone
Typically an LM pectin is used or this type of gel, because charged group are needed and LM pectins have enough free carboxyl groups
Sugar free jam usually contains a calcium gel
Endogenous enzymes
Enzymes that are naturally present in the plant
Use of pectin enzymes in food technology
Higher yields for fruit juices
Clarification of fruit juices
Production of tomato paste (done by endogenous pectin enzyms)
The four locations where pectinases act on pectin
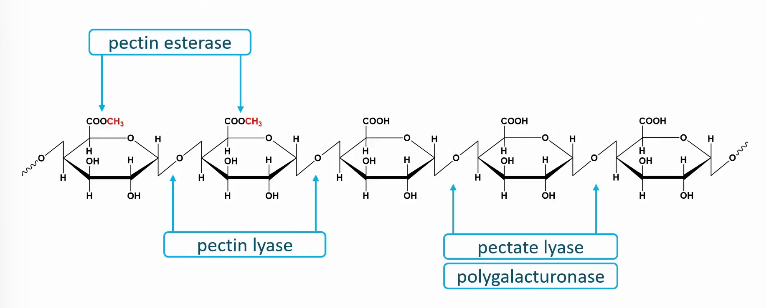
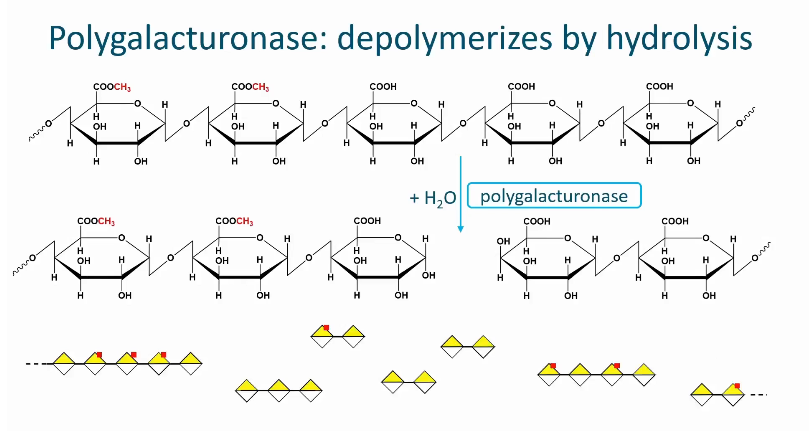
Polygalactaronase
A glycoside hydrolase
The enzymes hydrolyzes the glycosidic linkage next to a galactronic acid with a free carboxyl group (uses a water molecule)
Therefore, pectin with a low degree of methyl esterification is degraded by this enzyme
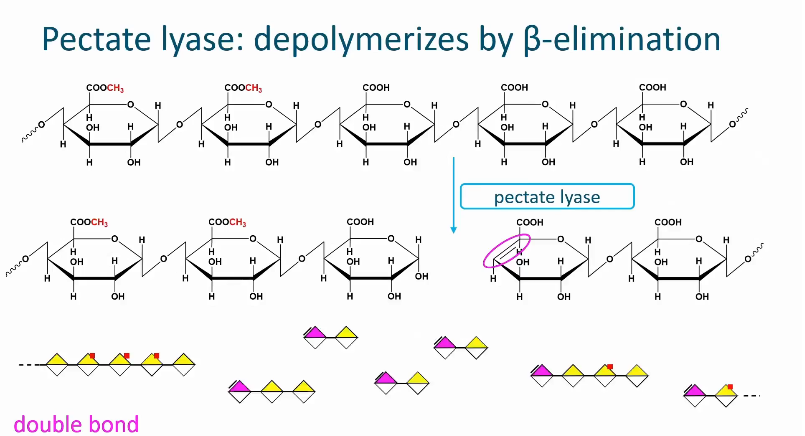
Pectate lyase
Cleaves the glycosidic linkage next to a galactronic acid with a free carboxyl group
Pectin with a low degree of methyl esterification is degraded by this enzyme.
Pectin lyase belongs to the lyase group meaning that is cleaves the glycosidic linkage by beta alumination
In this reaction, a double bond is introduced in a newly formed non-reducing chain end.
No water is introduced in this cleavage since it is not a hydrolysis reaction!
All reaction products have a double bond.
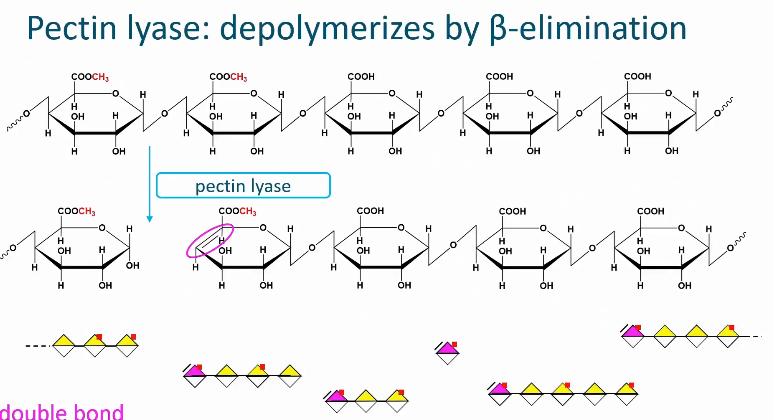
Pectin lyase
cleaves the glycosidic linkage next to a methyl esterified galactronic acid.
Therefore pectin with a high degree of methyl esterification is degraded by this enzyme
Belongs to the lyase group with means that it cleaves the glycosidic linkage by beta alumination
A double bond is introduced in a newly formed non-reducing chain end
With this reaction alum pectin is depolymerized into smaller oligosaccharides
All reaction products have a double bond
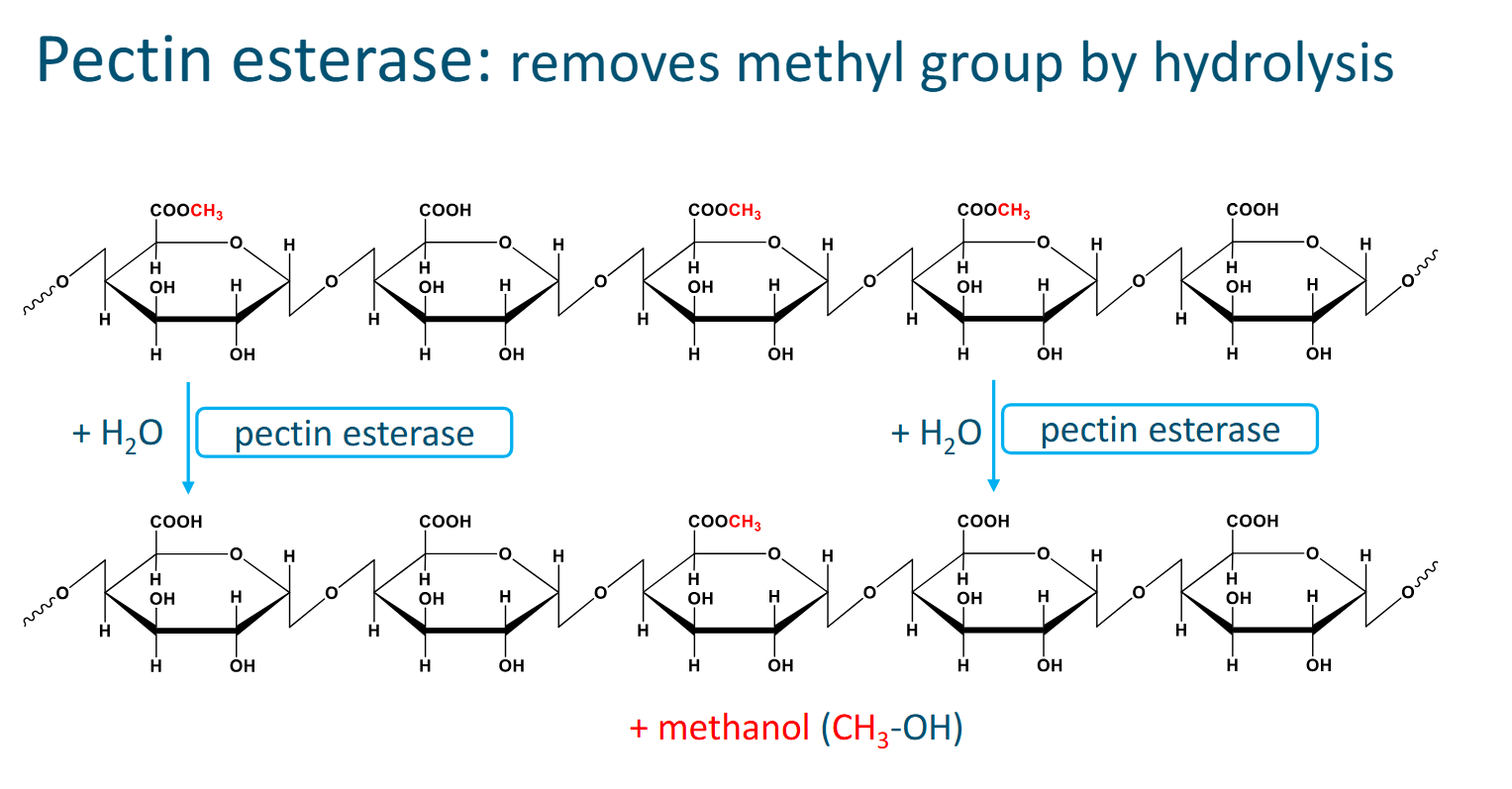
pectin esterase (or pectin methyl esterase)
Is an esterhydrolase
Very specific to the methyl ester group
Saponifies the astro bond, introducing water and releasing a methanol group.
What is left is the carboxyl group
Pectin esterase turns a pectin with a high degree of methyl esterification such as DM70 pectin, into a pectin with a low degree of methyl esterification such as DM30
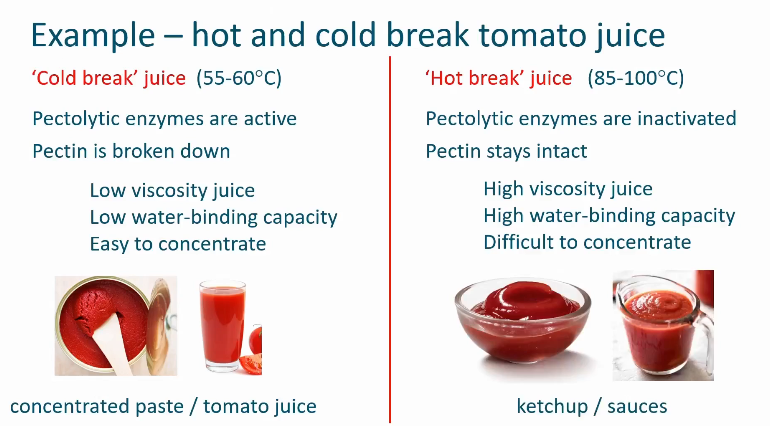
Cold break juice vs hot break juice
Roles of starch as additive in food
Thickening of soups, pie filling
Gelling: gum drops
Encapsulation: flavors
Crisping: fried snacks
What do starch granules look like?
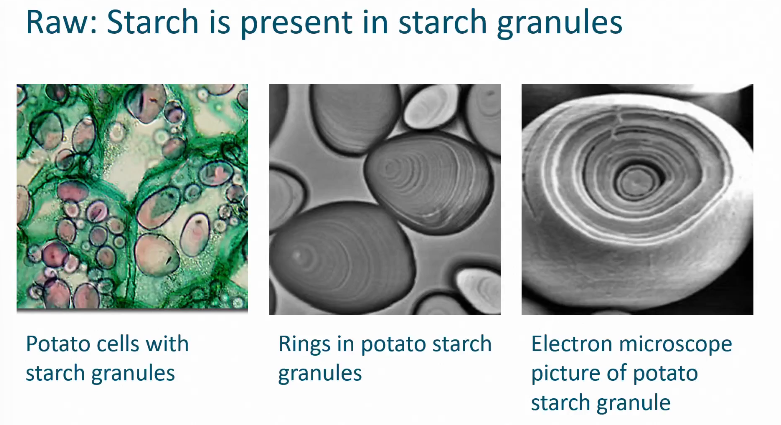
Composition of starch
Amylose
25-30% of starch
DP between 500-6000
Linear, alpha 1→4 linkage
Amylopectin
70-75% of strach
DP between 3×10^5-3×10^6
Branching, alpha 1→ 4 linkage for linear part. Alpha 1→ 6 for branched part
Organization of a starch granule
Crystalline area: highly branched and ordered = amylopectin
Anamorphous: amylose (less structured)
Hilum starch granule
the central point or origin of a starch granule, where its growth begins
Heating starch granule
Gelatinization
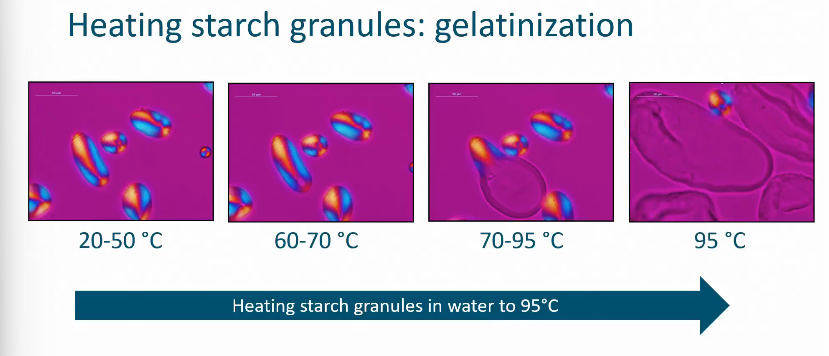
Gelatinization and pasting
Process is irreversible!
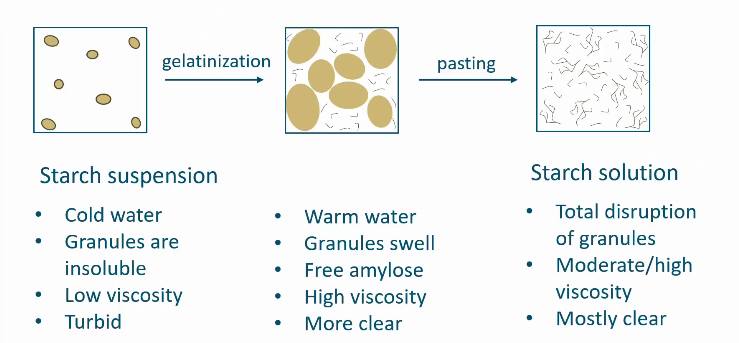
How do you monitor gelatinization in starch molecules
Measure and track viscosity
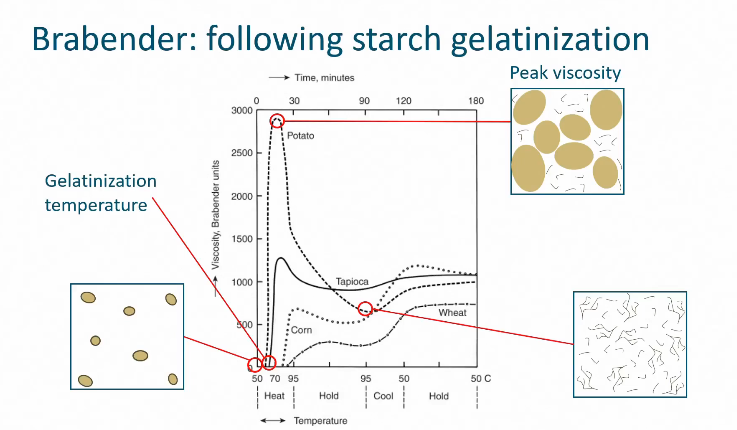
What is the increase of viscosity in starch called when cooling
Retrogradation
Is caused by reassociation of the starch molecules, mostly amylose molecules
Involves the formation of chain entanglements and crystallization of double helical aggregates
Retrogradation makes products stiff
It is a major factor in the staling of old bread and other bakery products
This process is reversible!
When heating a retrogredated product, the starch molecules disassociate again forming separate molecules
Applications in processing of gelatinizing starch: physical
Pregelatinized starch: dissolves without heat
Applications in processing of gelatinizing starch: Thin-boiling starch
Thin-boiling starch: reduced hot-paste viscosity (starch has been treated with acid to partly hydrolyze polysaccharides gives a low and uniform viscosity)
Applications in processing of gelatinizing starch: Cross-linked starch
Cross-linked starch: retains viscosity when heated/stirred (has been chemically treated to form cross-links between starch molecules)
Applications in processing of gelatinizing starch: Substituted starch
Substituted starch: reduced gelation/retrogradation (Starch molecules are substituted with extra groups, these groups hinder interaction between starch molecules)
Applications in processing of gelatinizing starch
Physical:
Pregelatinized starch
Chemical:
Thin-boiling starch
Cross-linked starch
Substituted starch
Amylases
Enzymes that hydrolyze glycosidic linkages in starch molecules
Alpha and beta amylase hydrolyze exclusively 1→ 4 linkages.
Glucoamylase splits both 1→4 linkages and 1→6 linkages (but much slower)
Isoamylase hydrolyzes the branched linkages (1→6)
Alpha amylase
Endoenzyme (cleaves in the middle of the chain)
Cleaves only 1→4 linkages.
A mixture of unbranched and branched malto-oligosaccharides with a DP of 2 to 6
This is also called dextrins
Beta amylase
Exoenzyme - Acts on the non-reducing chain end
Forms maltose & dextrin
Keeps going until stopped by an alpha 1→6 linkage
The released maltose is in the beta form
Glucoamylase
Exoenzyme - acts on the starch molecule from the non-reducing end.
Forms glucose
Can hydrolyze 1→ 4 and alpha 1→ 6 linkages but 1 to 6 is much slower
Therefore a complete hydrolysis can be done
Isoamylases
Exo-enzymes
Cleaves alpha 1→ 6 linkages
Product is relative long linear alpha 1→4 glucan chains
In practice is usually comined with glucoamylase
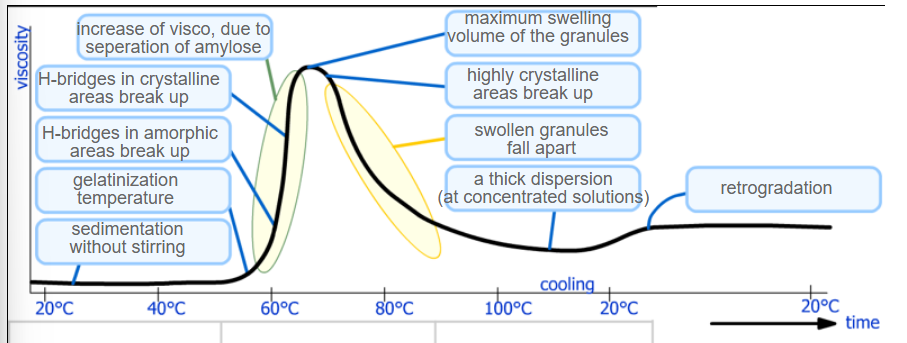
Steps in gelatinization of starch
Sedimentation without stirring
Gelatinization temperature (~60C)
H-Bridges in amorphic areas break up
H-bridges in crystalline areas break up
Increase of viscosity due to separation of amylose
Maximum swelling volume of the granules
Highly crystalline areas break up
Swollen granules fall apart
A thick dispersion
Retrogradation
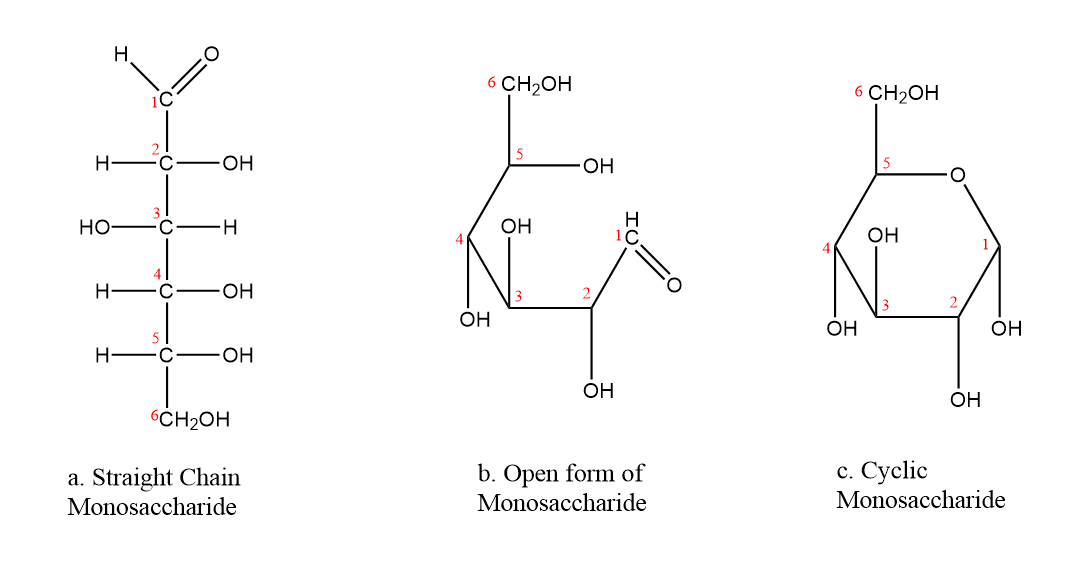
Aldose structure (open and closed)
Monomeric units of saccharose, lactose & maltose
Saccharose: glucose + fructose
Lactose: Galactose + glucose
Maltose: Glucose + glucose
What is an invert sugar?
liquid sweetener made by breaking down sucrose (table sugar) into equal parts of its simple sugar components, glucose and fructose, using acid or enzymes
Done by hydrolyzing sucrose by leaving its a-1→2 glycosidic bond
Mode of action lactase