Glycolysis: Prep phase, payoff phase, fermentation, pyruvate dehydrogenase, TCA
1/78
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
79 Terms
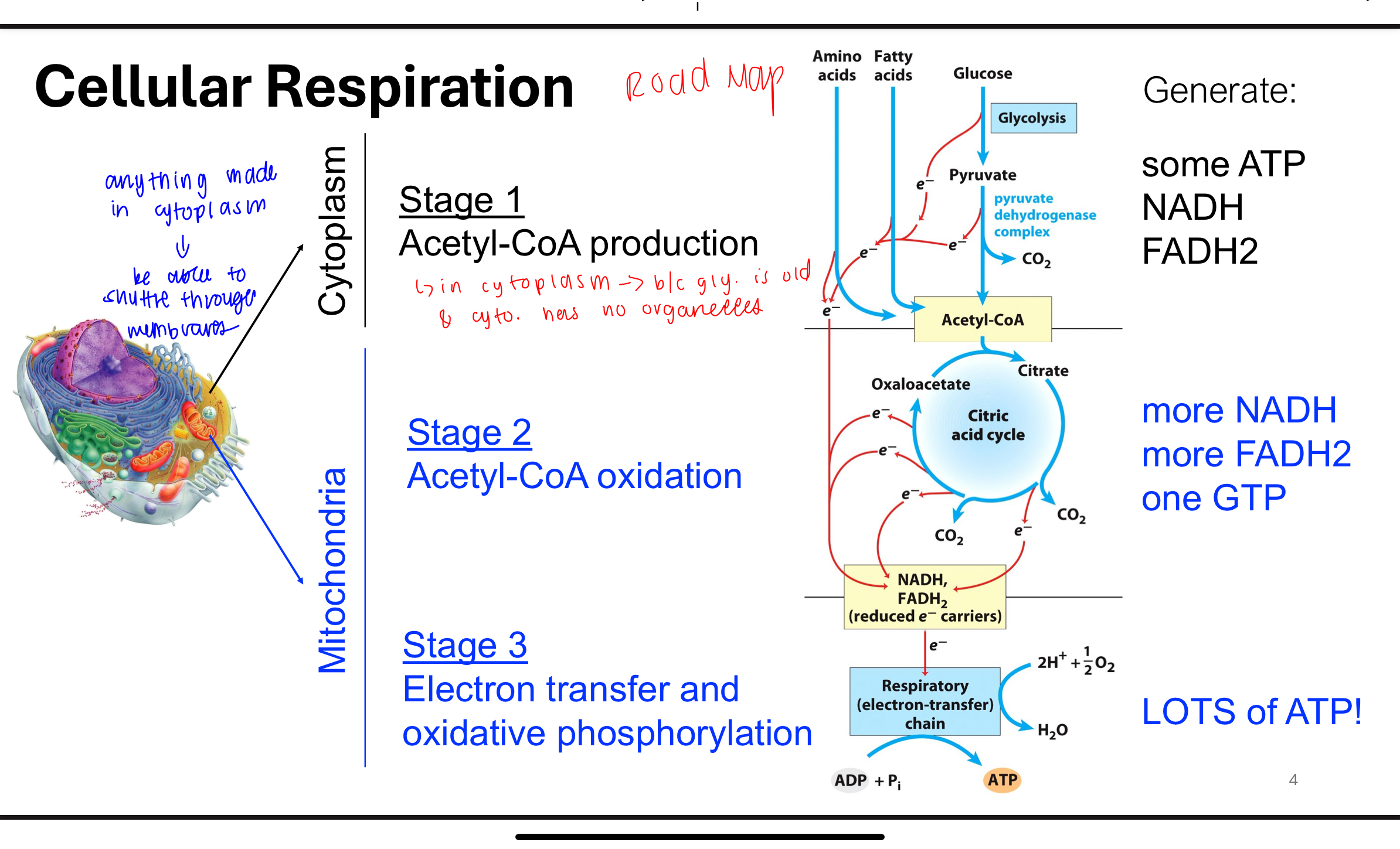
Q: What is the overall goal of cellular respiration, and how is free energy captured?
A: The goal of cellular respiration is to oxidize glucose (a sugar) into carbon dioxide and water, releasing energy.
This energy is stored in the form of ATP and NADH, with some lost as heat. The process includes three stages:
1) Acetyl-CoA production,
2) Acetyl-CoA oxidation (Krebs cycle),
3) Electron transport and oxidative phosphorylation.
Q: What does the term 'glycolysis' mean and what is its role?
A: "Glycolysis" means "sugar splitting." It's the first pathway in cellular respiration where glucose (a 6-carbon sugar) is broken down into two 3-carbon molecules of glyceraldehyde-3-phosphate (G3P). It occurs in the cytoplasm and produces small amounts of ATP and NADH.
Q: Why is glycolysis considered ancient and nearly universal?
A: Glycolysis is found in almost all organisms and does not require oxygen, making it a key pathway for energy production in anaerobic organisms, red blood cells, and during intense muscular activity.
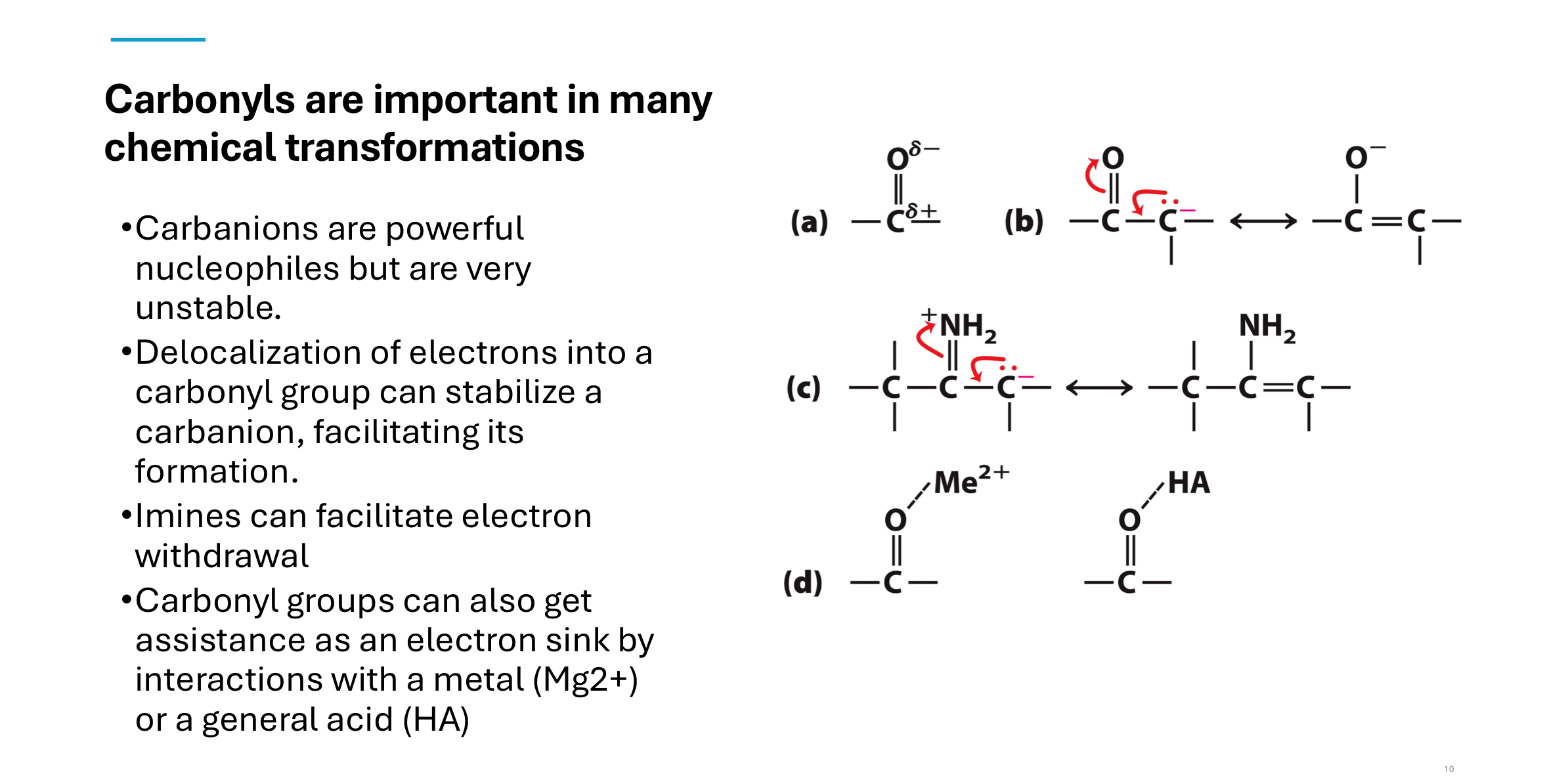
Q: Why are carbonyls important in biochemical reactions?
A: Carbonyl groups stabilize carbanions, which are otherwise unstable but essential nucleophiles.
This stabilization occurs via electron delocalization, interaction with metals (Mg²⁺), or proton donors (acids).
Imines can also assist in withdrawing electrons.
many kinase enzymes have Mg2+ in the active site of the cofactor? Why?
Helps balance the negative charges of the ATP that are found in the active site
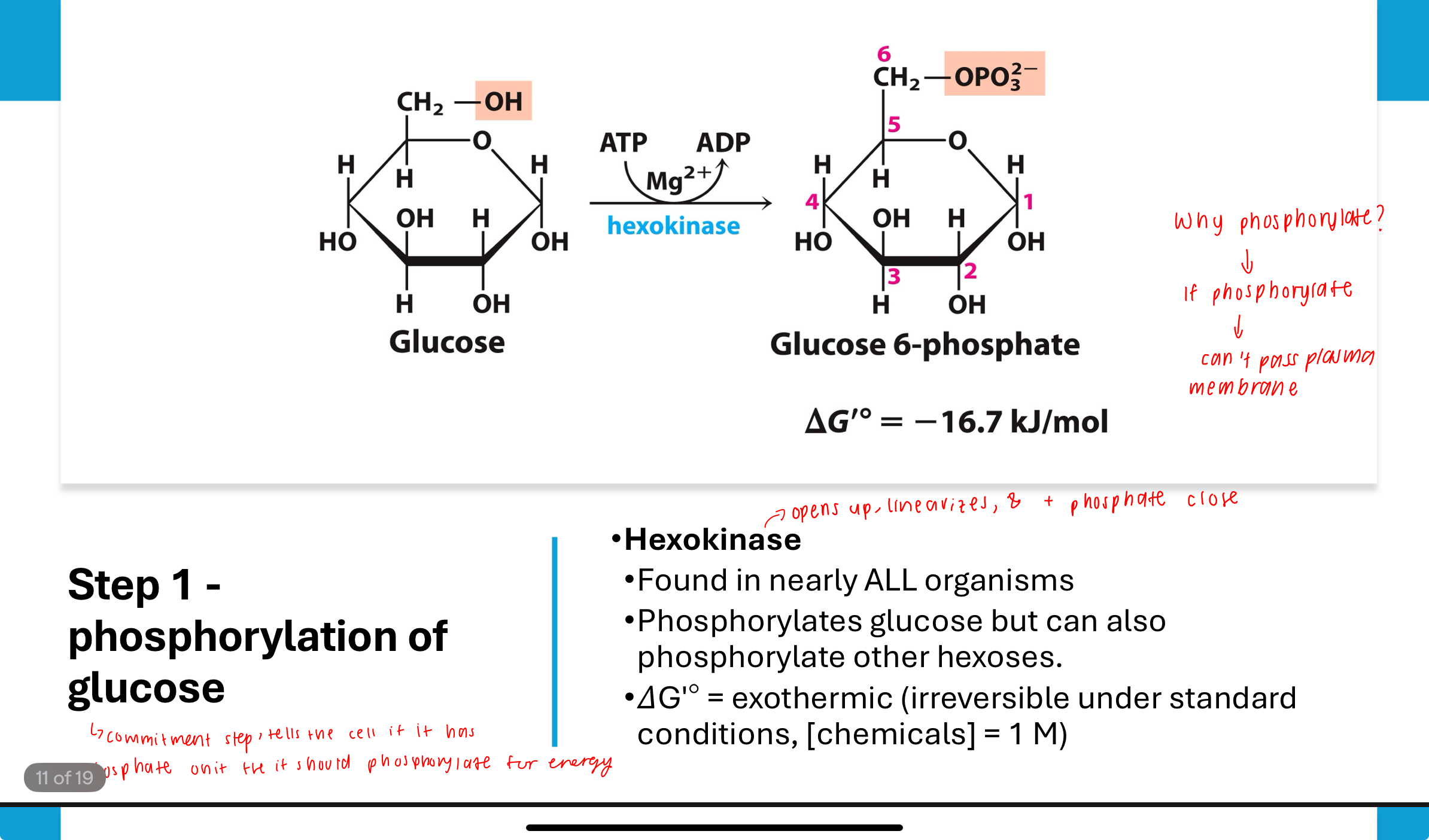
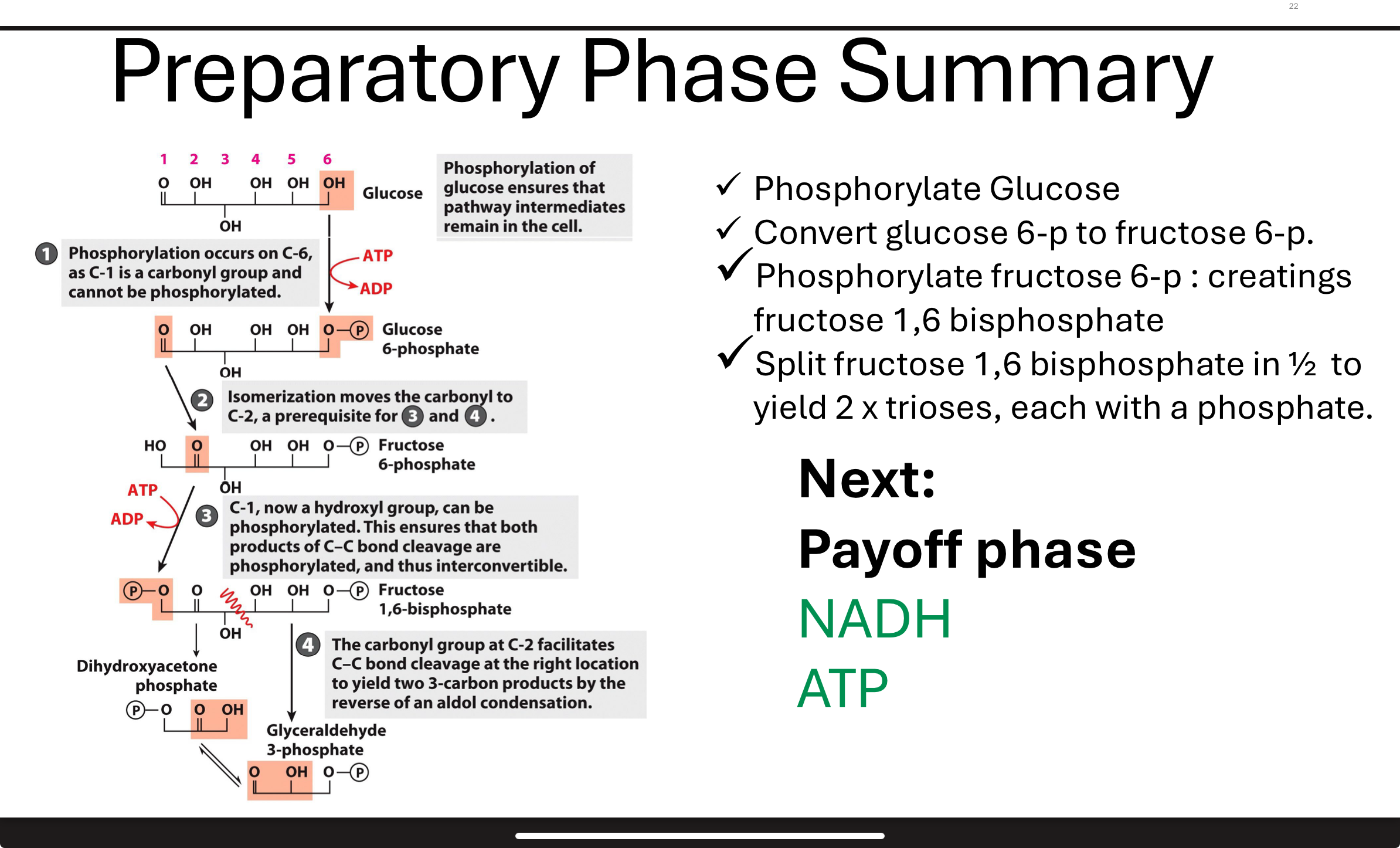
What occurs in Step 1 of glycolysis?
Phosphorylation of glucose (commitment step, tells the cell it has phosphate unit)
Glucose is phosphorylated by hexokinase using ATP to form glucose-6-phosphate. This step is exothermic and essentially irreversible (ΔGʹ° is strongly negative). It traps glucose in the cell and activates it for further reactions bc phosphorylation prevents glucose from passing through cell membrane—negatively charged (cannot leave cell).
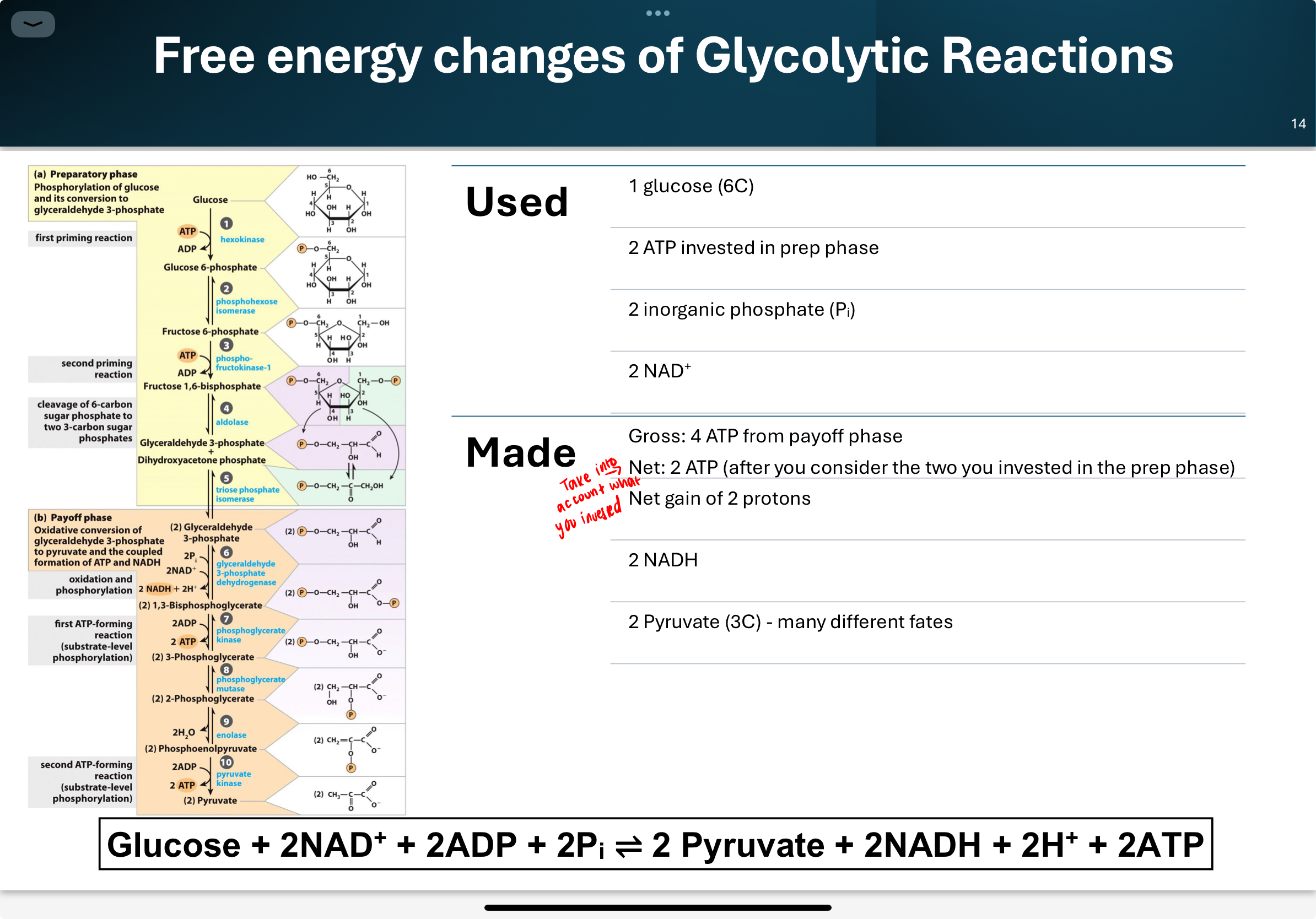
Q: What happens during the investment phase of glycolysis (steps 1–5)?
• Energy of ATP is invested to raise the free energy of the
intermediates.
• In one round of glycolysis, 1 molecule of glucose is
converted to 2 identical products (G3-P) before entering the next phase (steps 6-10).
• Glucose, 6 carbon sugar (hexose) →2 x 3 carbon sugars (trioses)
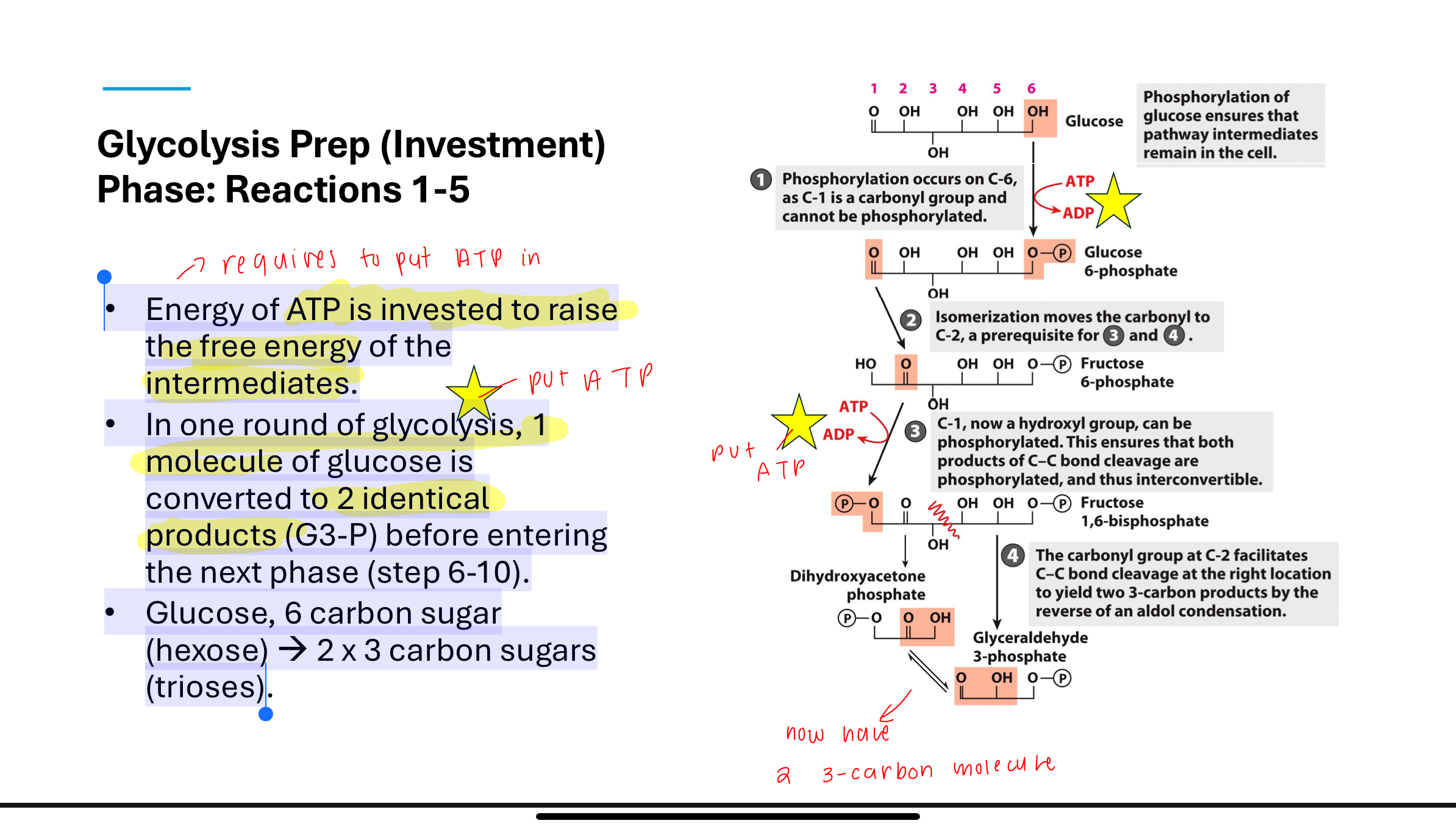
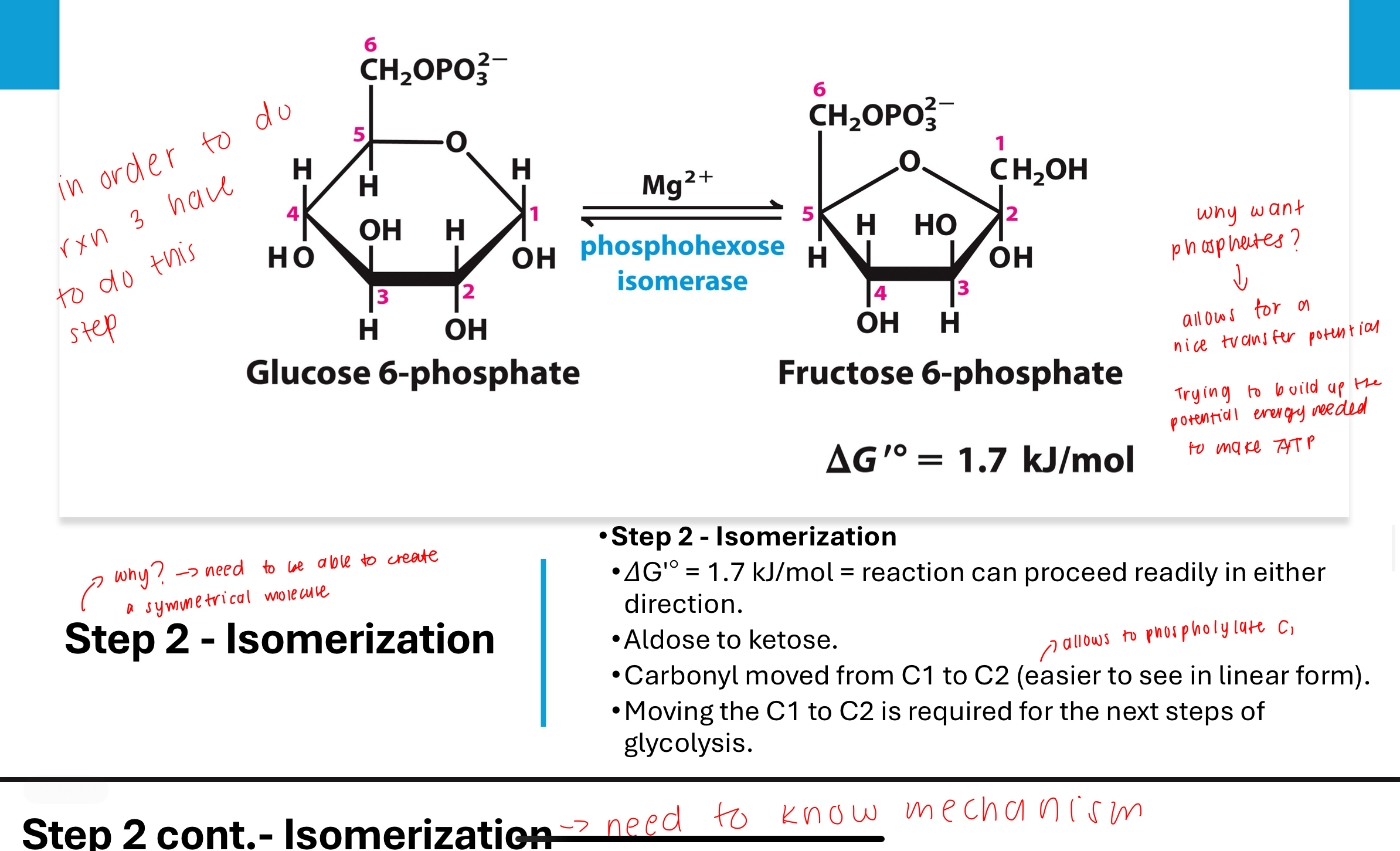
What happens in Step 2 of glycolysis?
the purpose is to create a symmetrical molecule
in order to do reaction 3 have to do this step
Glucose-6-phosphate is isomerized to fructose-6-phosphate by phosphohexose isomerase. This converts an aldose to a ketose, making it suitable for subsequent phosphorylation and cleavage. ΔGʹ° is close to zero, so the reaction is reversible.
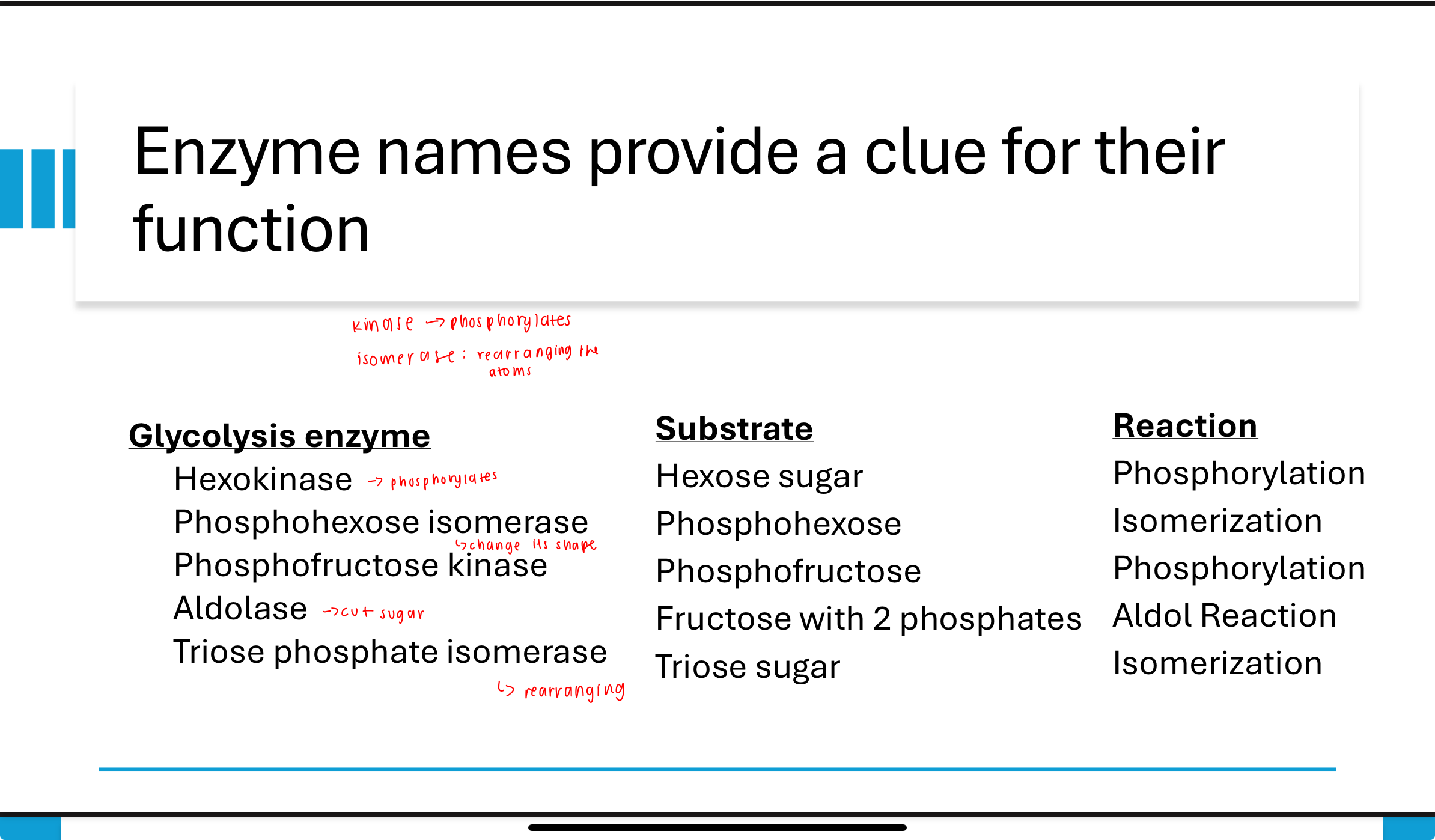
Q: How do enzyme names help identify their function in glycolysis?
A: Enzyme names often describe their action:
Hexokinase: phosphorylates hexoses
Phosphohexose isomerase: isomerizes phosphohexoses
Phosphofructokinase: phosphorylates fructose
Aldolase: performs aldol cleavage
Triose phosphate isomerase: isomerizes trioses
kinase- phosphorylates
isomerase- rearranging the atoms (changes its shape)
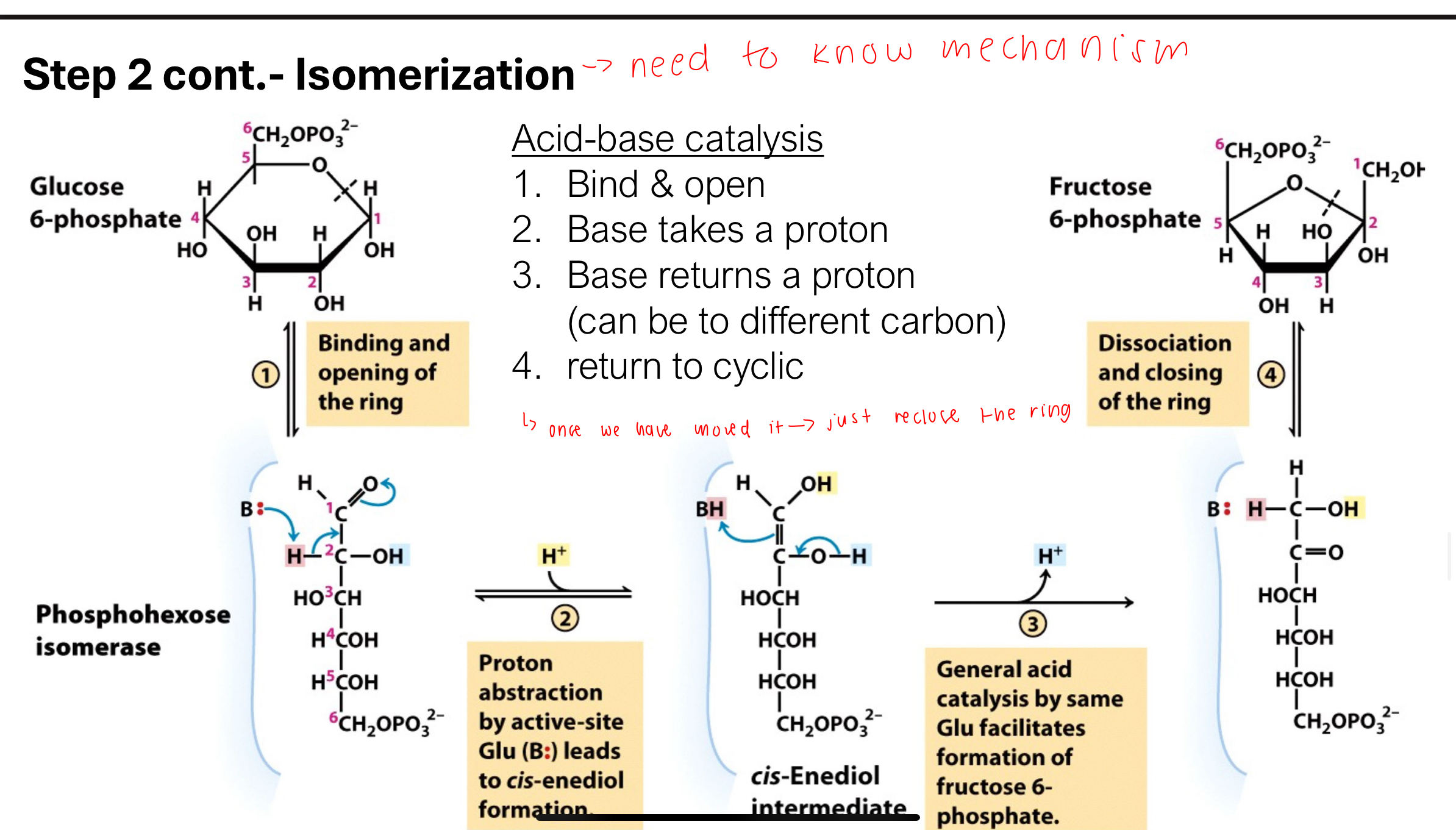
What is the mechanism of the isomerization in Step 2?
A: Acid-base catalysis is involved:
The ring opens
A base removes a proton
A new proton is added to a different position
The molecule re-cyclizes
What happens in Step 3 of glycolysis?
A second ATP is used to phosphorylate fructose-6-phosphate into fructose-1,6-bisphosphate by phosphofructokinase (PFK).
This step commits the molecule to glycolysis and is highly exothermic (essentially irreversible).
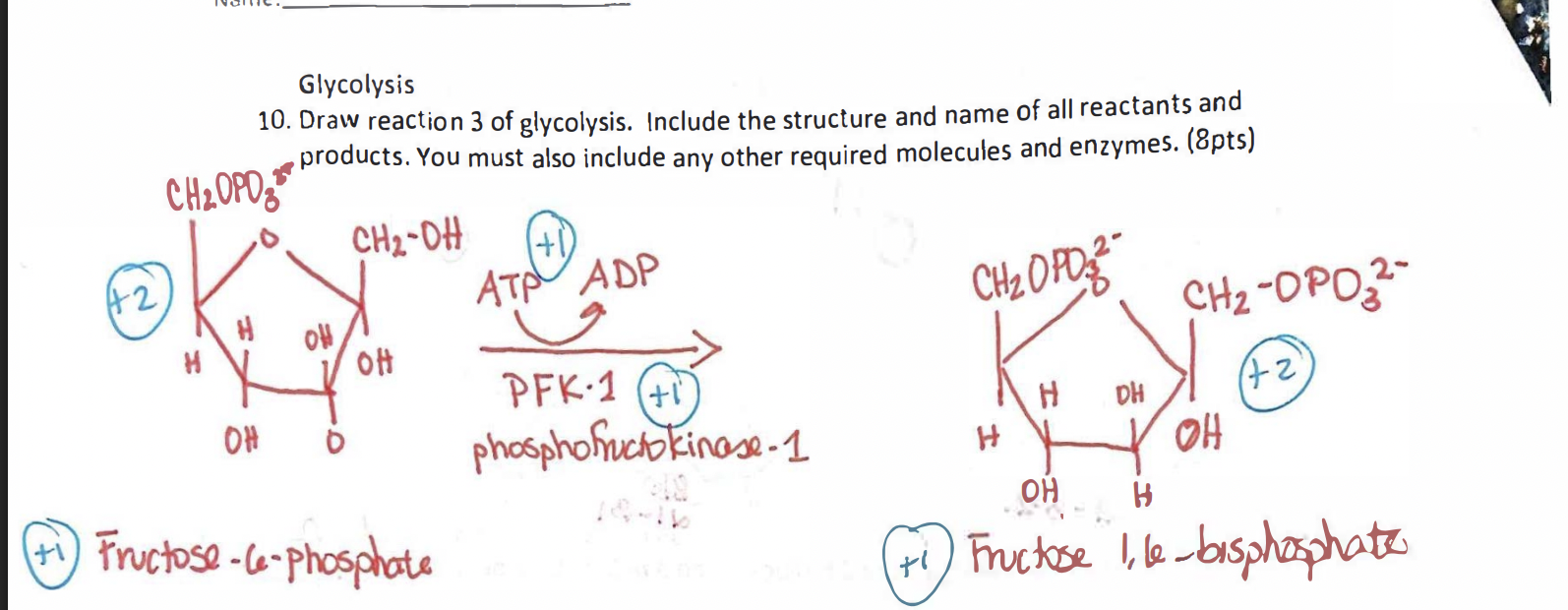
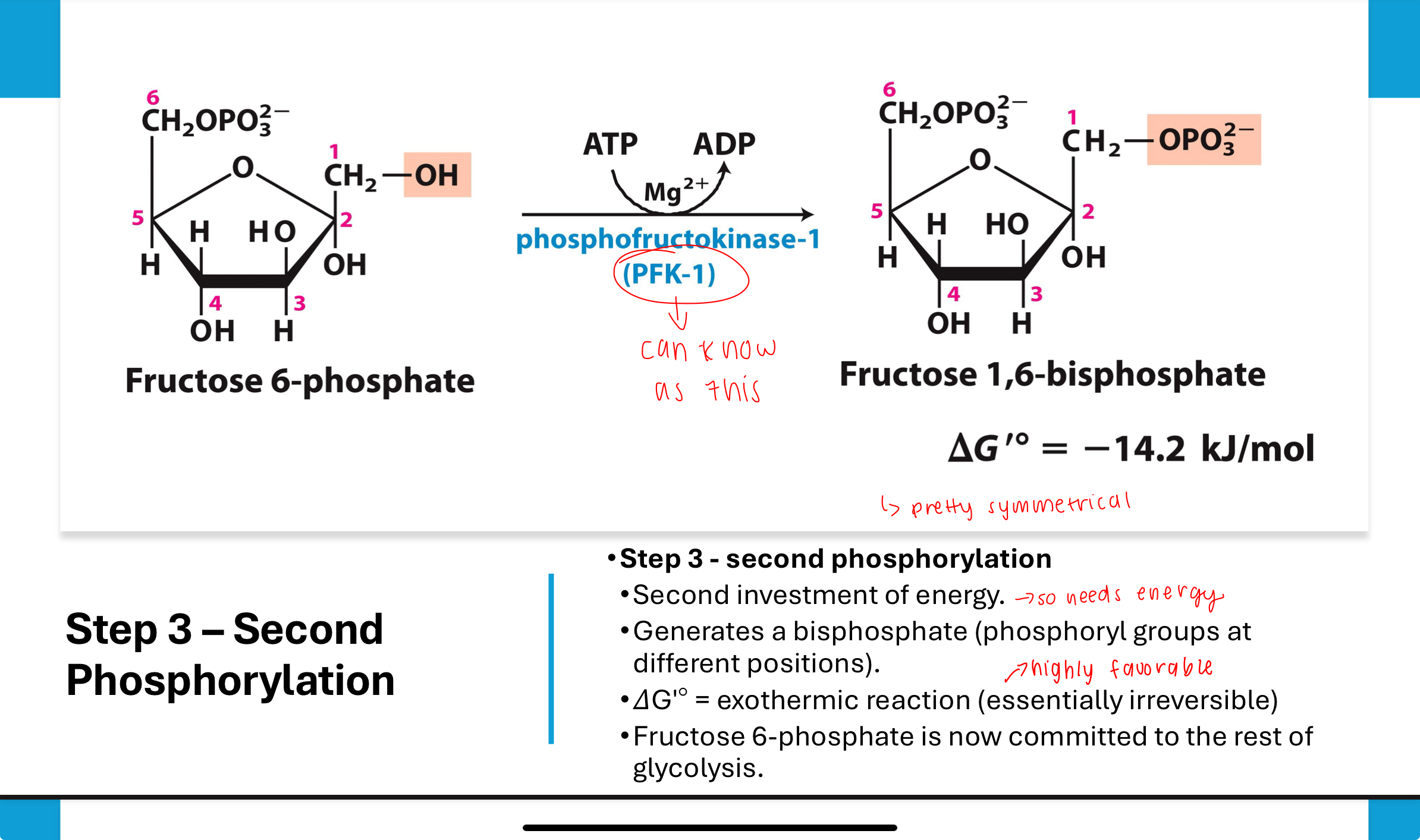
What is the purpose of Step 3 in glycolysis?
This step commits the sugar to glycolysis by creating a molecule that can no longer be used in other pathways. It also primes it for cleavage into two trioses in Step 4.
What occurs in Step 4 of glycolysis?
Fructose-1,6-bisphosphate is cleaved by aldolase into two 3-carbon sugars: dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (G3P).
Though ΔGʹ° is positive, the actual ΔG is close to zero in cells due to product removal. Strongly positive
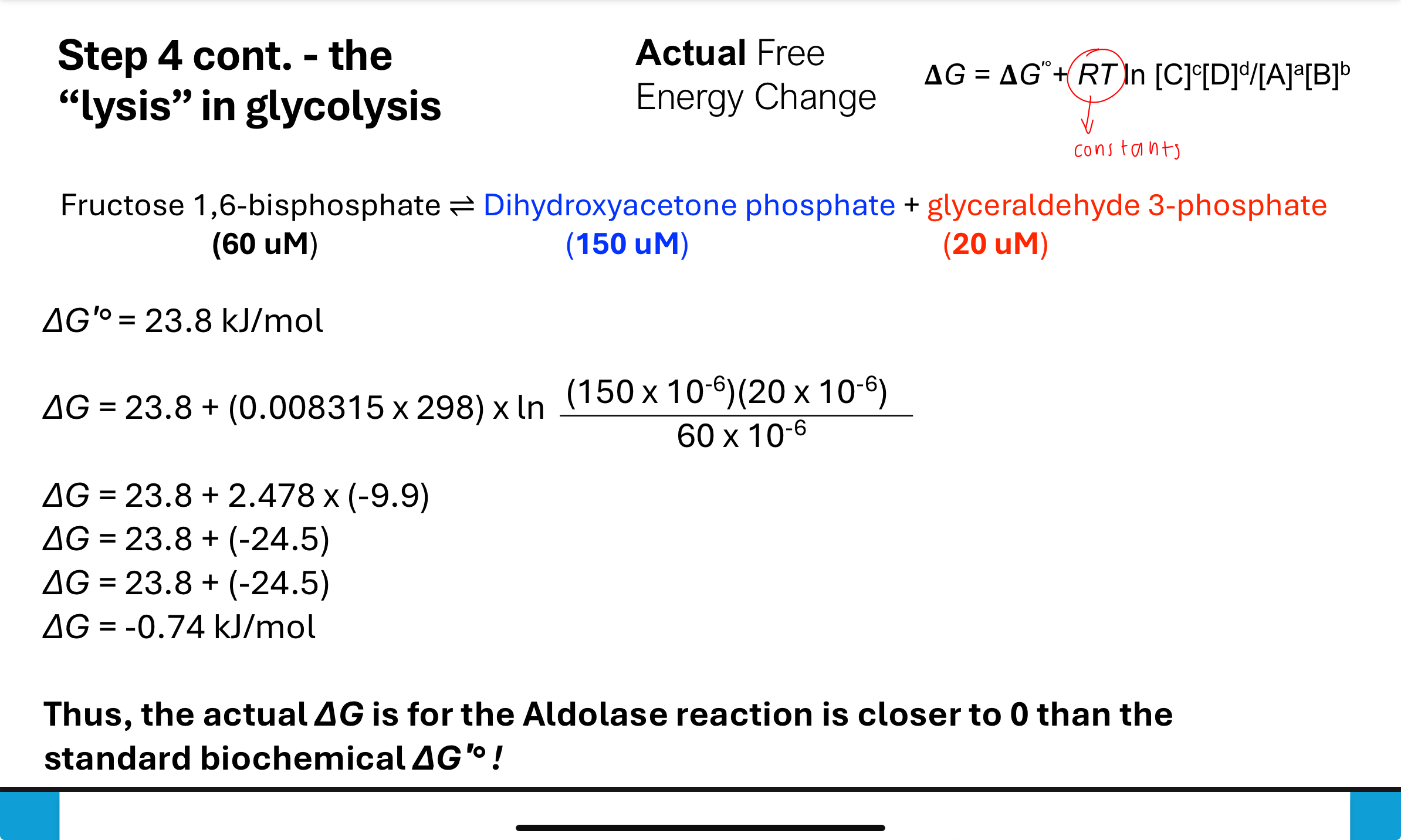
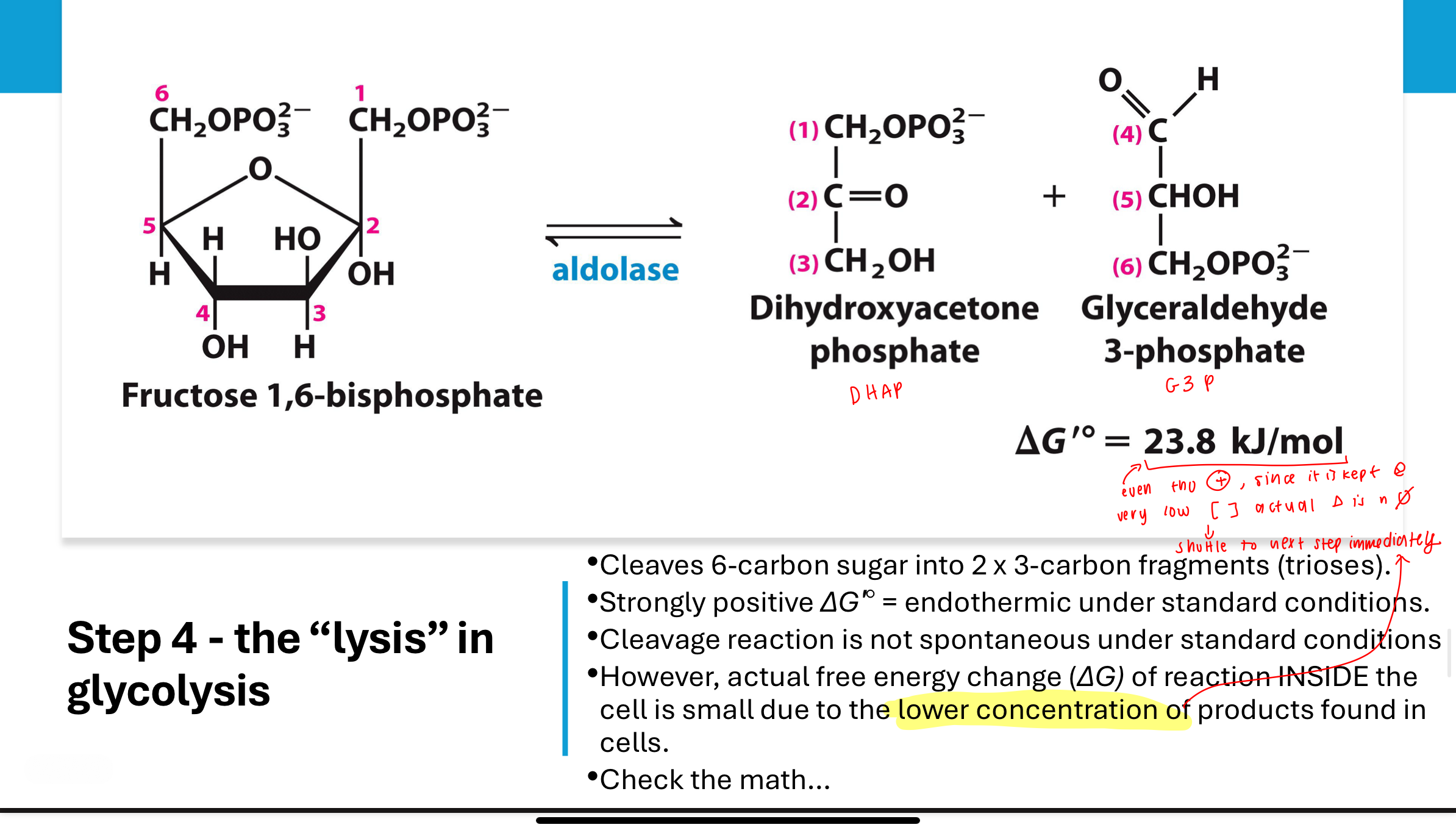
How does cellular concentration affect ΔG in Step 4?
Despite a standard ΔGʹ° of +23.8 kJ/mol, the actual ΔG becomes approximately -0.74 kJ/mol in the cell due to low concentrations of products. This makes the reaction nearly reversible under cellular conditions.
What happens in Step 5 of glycolysis?
Triose phosphate isomerase converts DHAP into G3P, so both products of Step 4 enter the payoff phase as identical 3-carbon intermediates.
This ensures energy recovery from both halves of the glucose molecule.
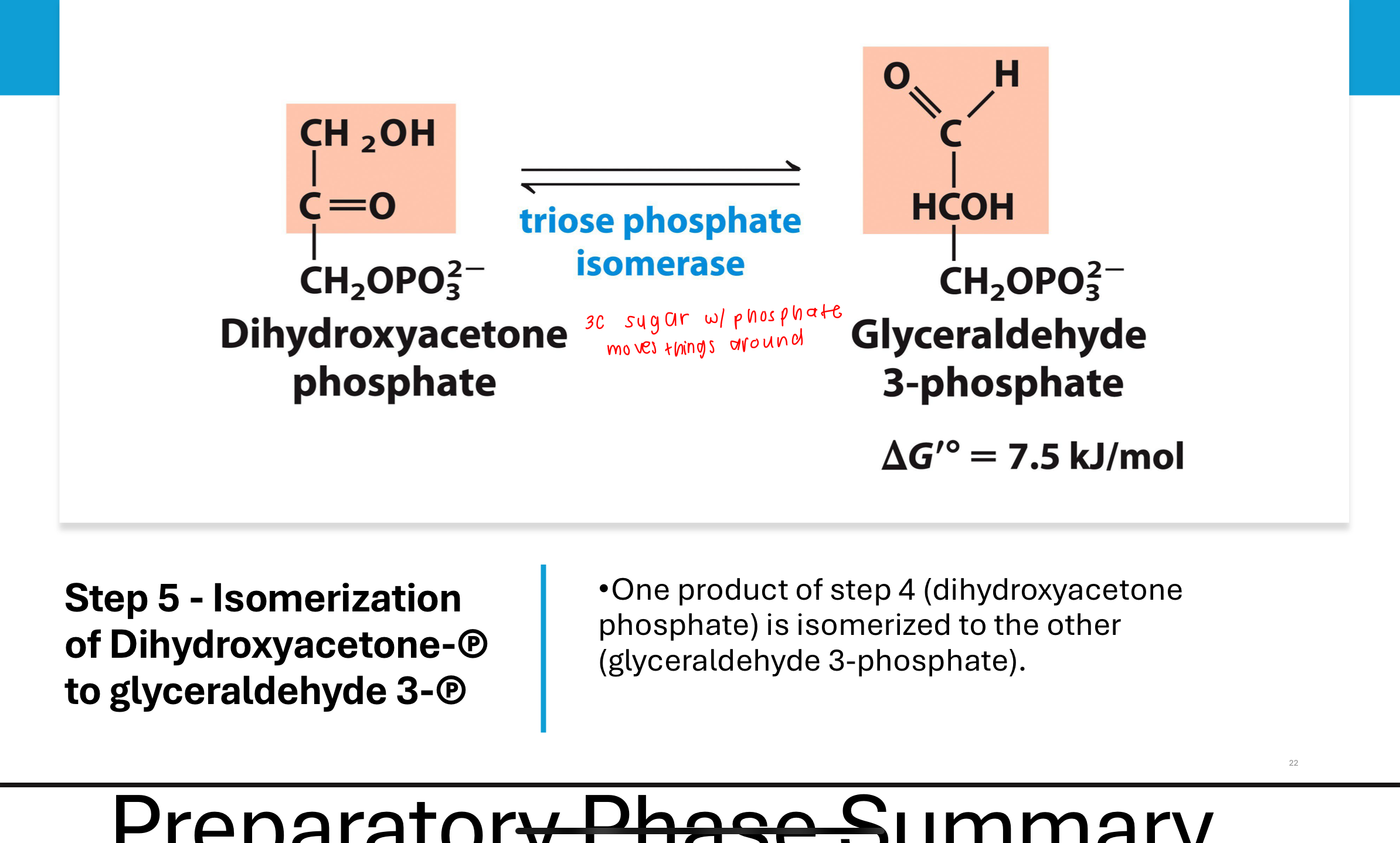
prep phase summary glycolysis
What is the overall purpose of the payoff phase in glycolysis?
The payoff phase (Steps 6–10) generates energy in the form of ATP and NADH. After glucose is split into two 3-carbon trioses, each undergoes a series of reactions to form pyruvate, producing a total of 4 ATP (gross), 2 NADH, and 2 pyruvate per glucose.
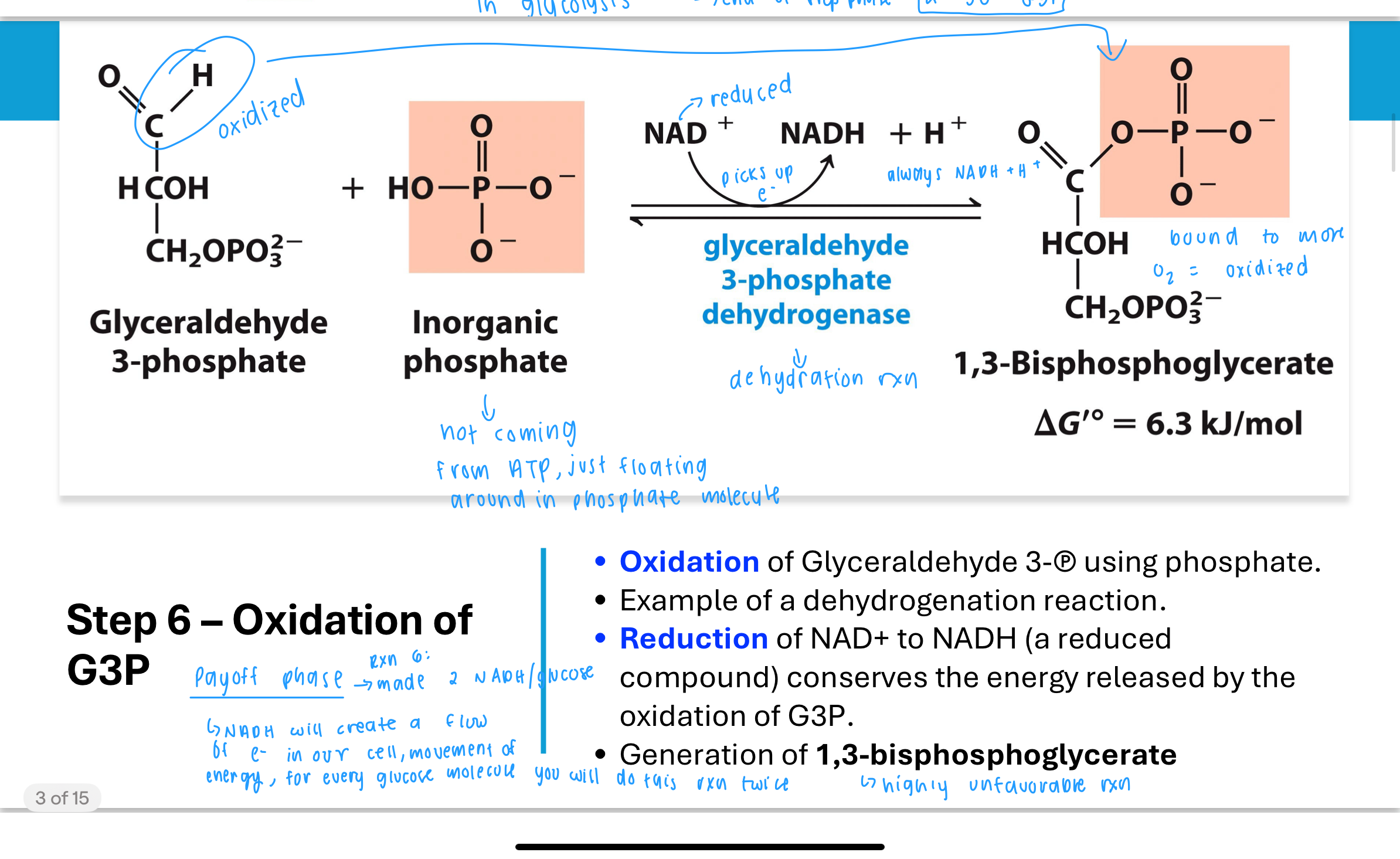
What happens in Step 6 of glycolysis?
In Step 6, the enzyme glyceraldehyde-3-phosphate dehydrogenase catalyzes the oxidation of G3P.
This reaction uses an inorganic phosphate (Pi) and reduces NAD⁺ to NADH, forming 1,3-bisphosphoglycerate (1,3-BPG).
This is a classic example of a dehydrogenation reaction. Importantly, the oxidation of G3P releases energy, and instead of losing it as heat, the cell captures this energy in the formation of a high-energy phosphate bond in 1,3-BPG and in the NADH, which will be used later to generate more ATP in the electron transport chain.
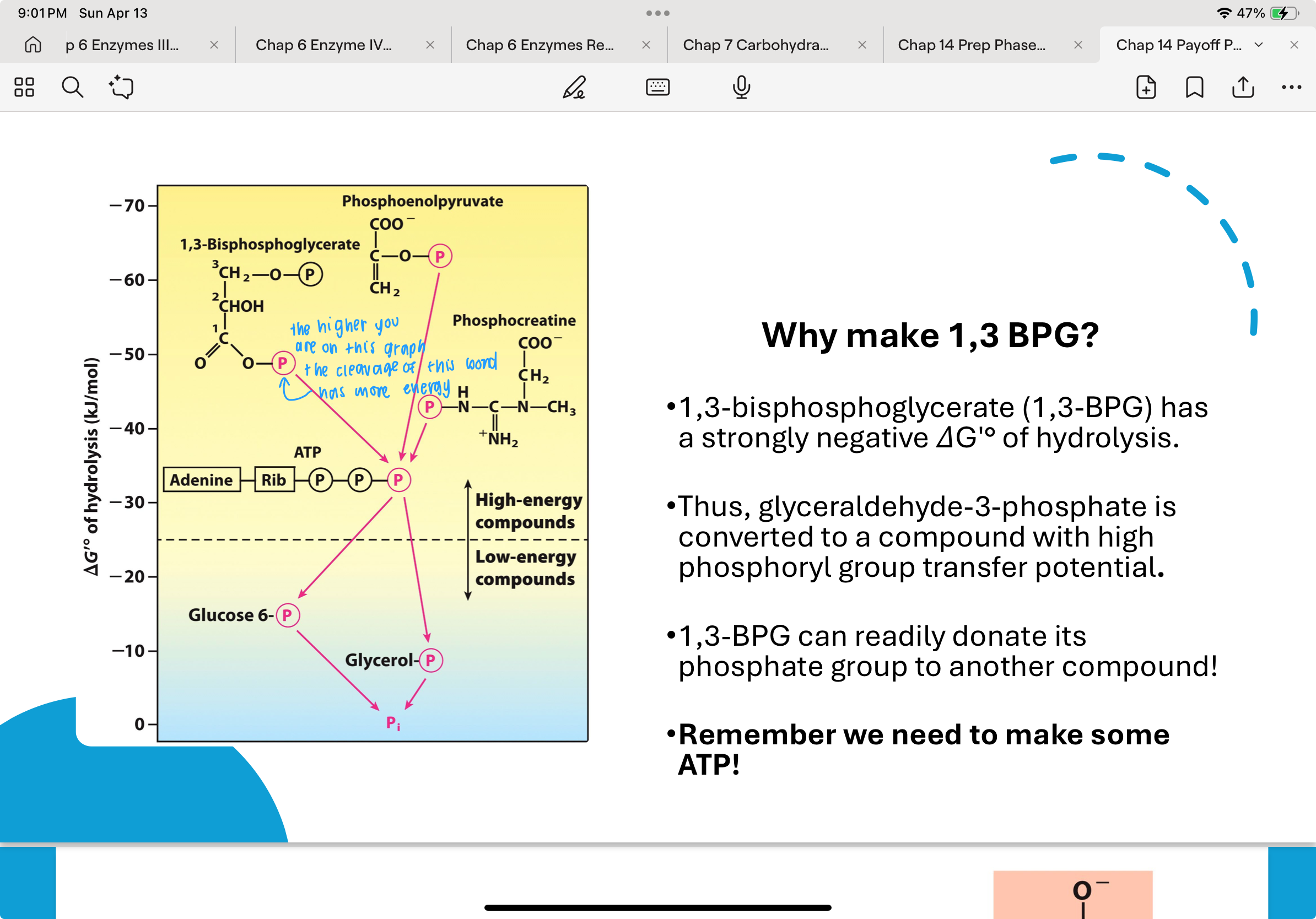
Why make 1,3 BPG?
The compound 1,3-bisphosphoglycerate (1,3-BPG) has a very high phosphoryl group transfer potential, meaning it can easily transfer its phosphate to another molecule, like ADP.
This is due to its extremely negative ΔGʹ° of hydrolysis. By converting G3P to 1,3-BPG, the cell temporarily stores energy in a form that can be used to produce ATP in the next step.
What happens in Step 7 of glycolysis, and how is ATP produced?
In Step 7, the enzyme phosphoglycerate kinase transfers the high-energy phosphate from 1,3-bisphosphoglycerate to ADP, forming ATP and 3-phosphoglycerate (3PG).
This is an example of substrate-level phosphorylation, a process where ATP is generated directly from a high-energy substrate without the need for the electron transport chain.
The reaction is exothermic and highly favorable due to the large negative free energy change associated with breaking the bond in 1,3-BPG.
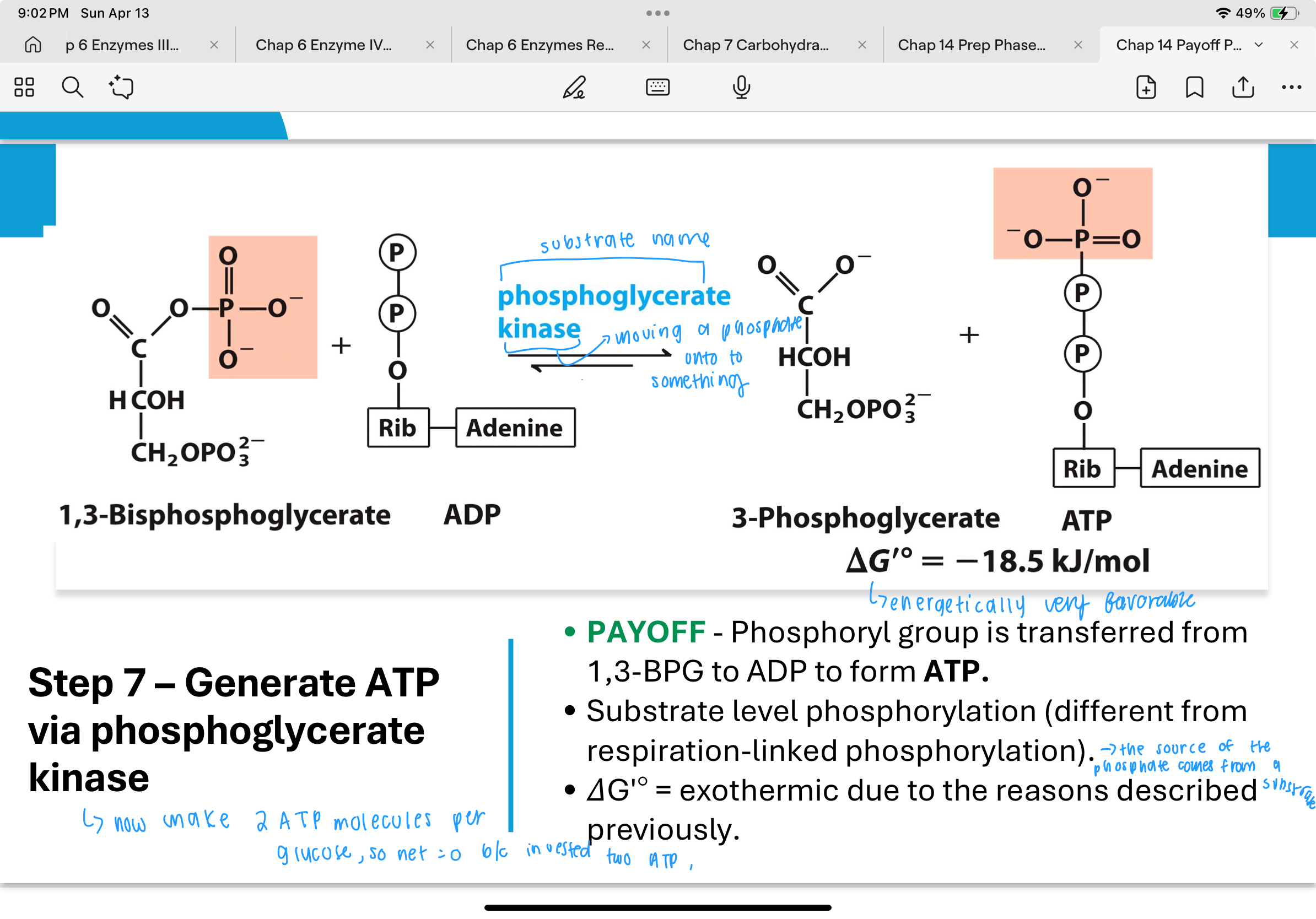
How are Steps 6 and 7 of glycolysis energetically connected?
Steps 6 and 7 are coupled reactions — the energy released from the oxidation of G3P in Step 6 is not wasted but immediately used in Step 7 to form ATP.
The sum of these two reactions:
G3P + NAD⁺ + Pi + ADP ⇌ 3PG + NADH + ATP + H⁺
has an overallΔGʹ° of about -12 kJ/mol, which ensures the reactions proceed efficiently and drive glycolysis forward.
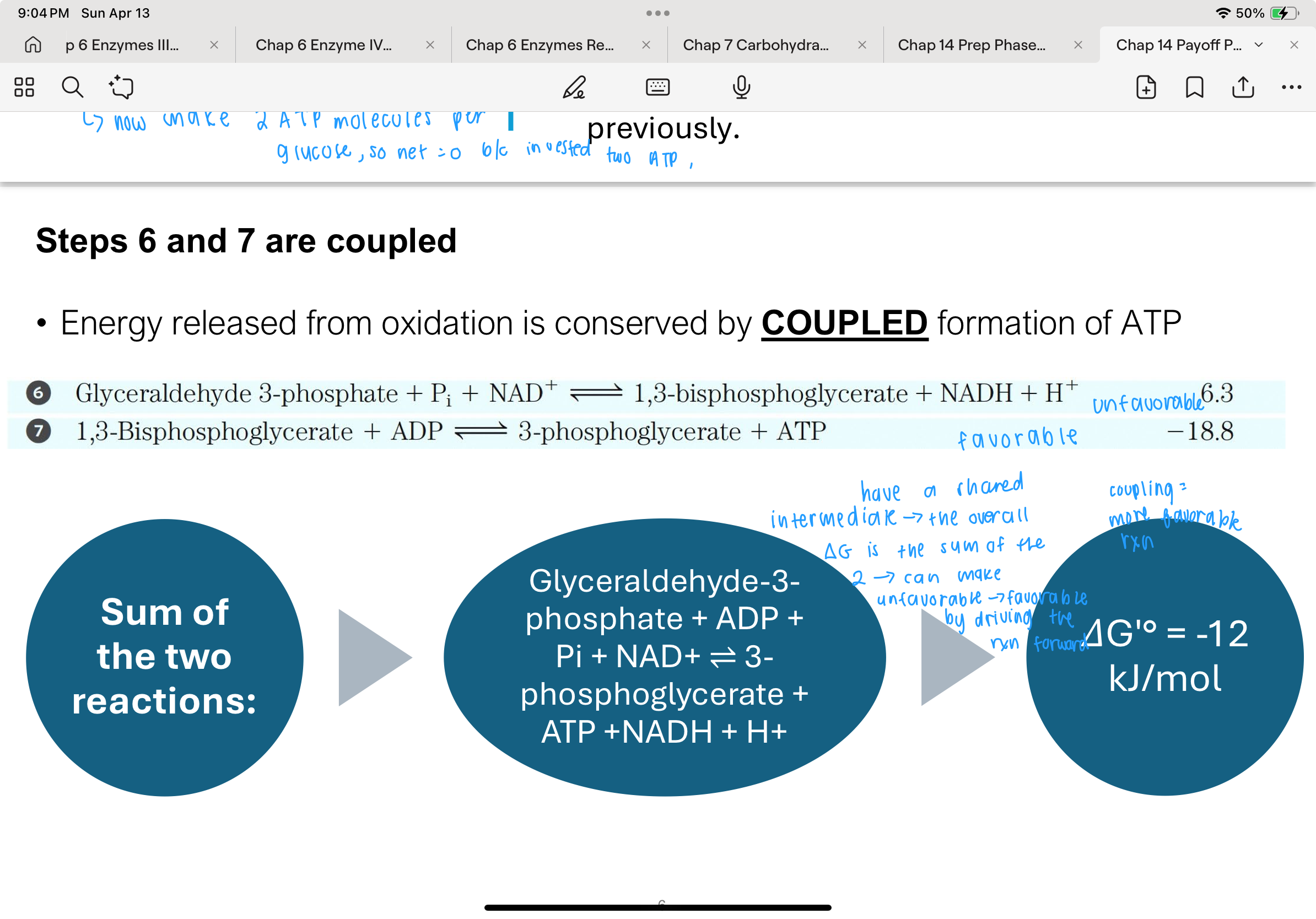
What is the function of Step 8 in glycolysis?
Step 8 involves the enzyme phosphoglycerate mutase, which shifts the phosphate group on 3-phosphoglycerate from the 3rd carbon to the 2nd carbon, producing 2-phosphoglycerate (2PG).
This rearrangement is essential because only 2PG — not 3PG — can undergo the dehydration reaction in the next step.
The rearrangement helps set up the molecule for the high-energy transformation to phosphoenolpyruvate.
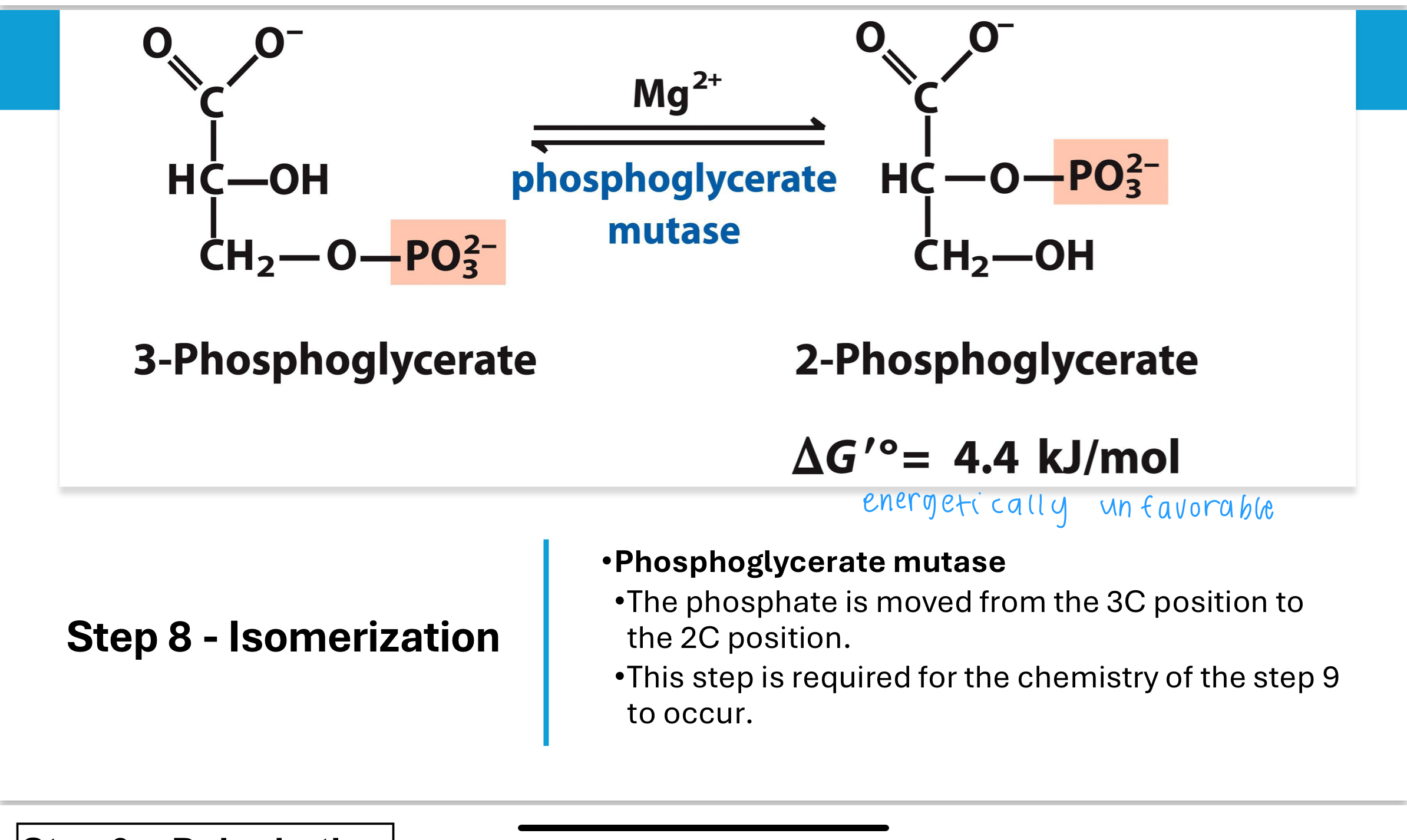
What happens in Step 9 of glycolysis, and why is it necessary?
In Step 9, the enzyme enolase catalyzes the removal of water (dehydration) from 2-phosphoglycerate, creating phosphoenolpyruvate (PEP).
This reaction involves eliminating water between carbons 2 and 3, forming a double bond that dramatically increases the molecule's phosphoryl group transfer potential.
Without first converting 3PG to 2PG (Step 8), this dehydration would not be chemically possible.
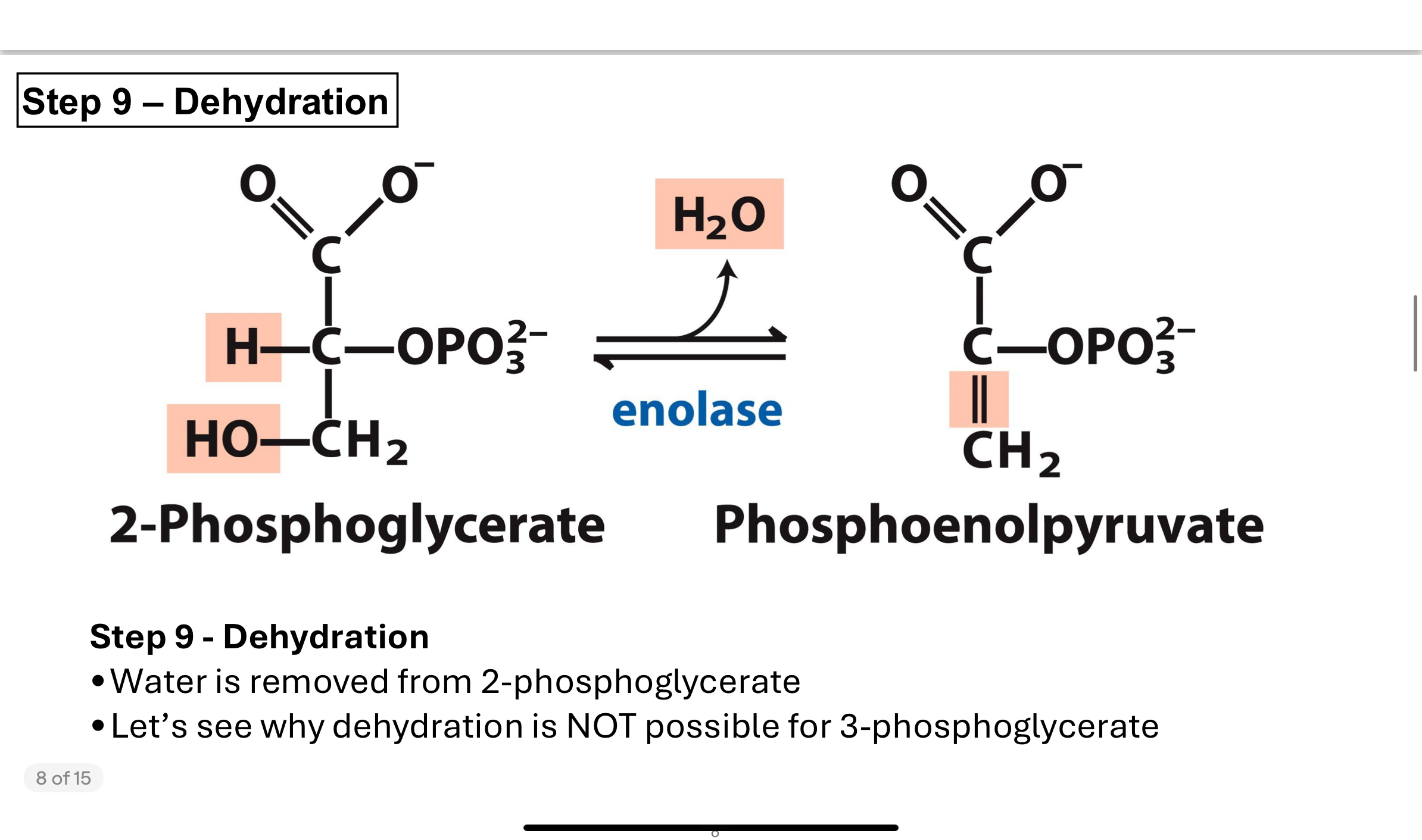
Why does the cell go through the extra steps to make phosphoenolpyruvate (PEP)?
PEP has one of the highest phosphoryl transfer potentials of any biological molecule.
Its hydrolysis is extremely exergonic (very negative ΔGʹ°), which means it can very efficiently donate its phosphate to ADP to form ATP.
Making PEP from 2PG allows the cell to produce another molecule of ATP in the final step of glycolysis.
What occurs in Step 10 of glycolysis, and how is ATP generated again?
In Step 10, the enzyme pyruvate kinase catalyzes the transfer of a phosphate from PEP to ADP, producing ATP and pyruvate.
This is another example of substrate-level phosphorylation.
The reaction is highly favorable because pyruvate undergoes tautomerization — it shifts between its enol and keto forms, which stabilizes the product and helps drive the reaction forward with a strongly negative ΔGʹ°.
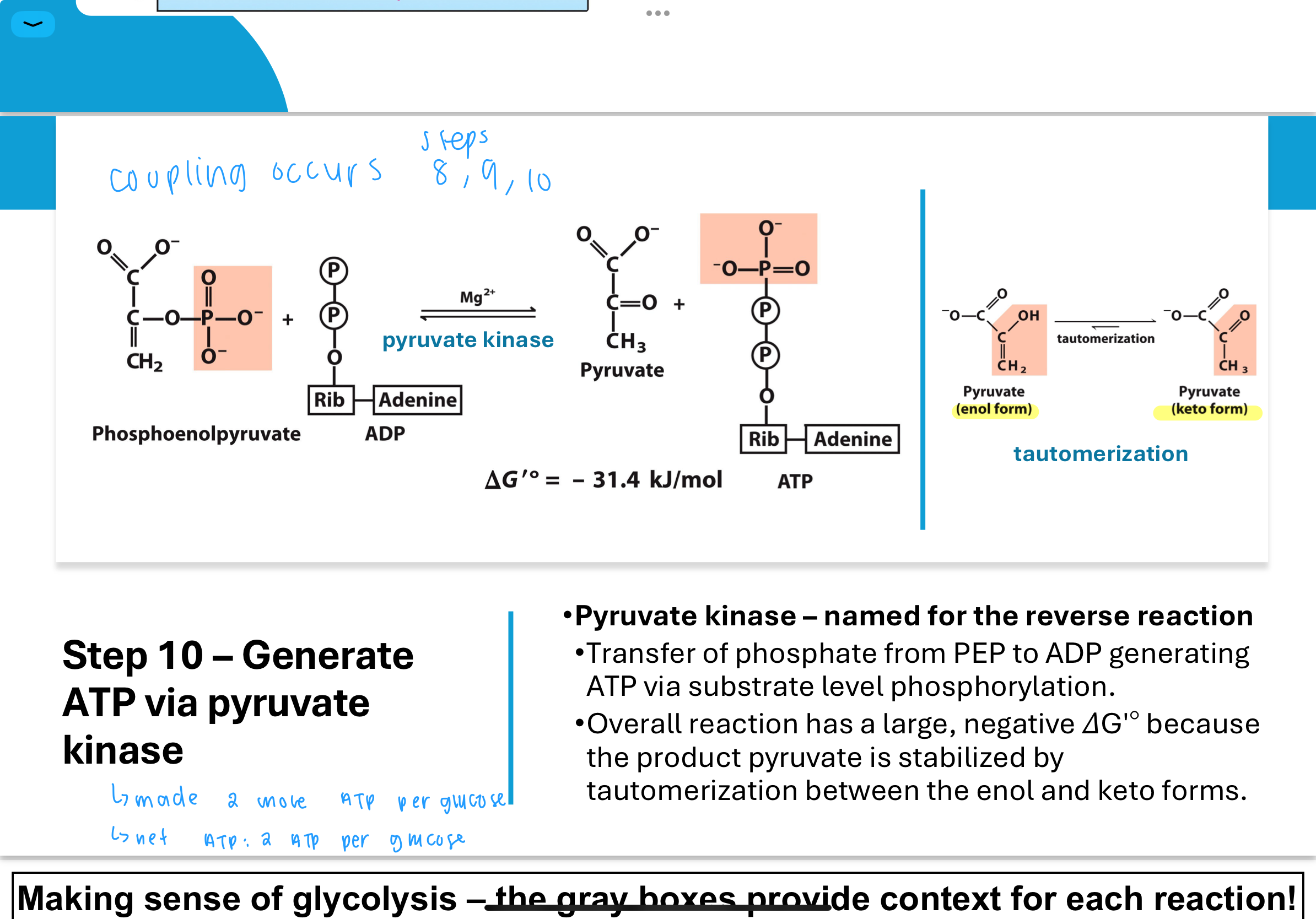
What is tautomerization, and how does it drive Step 10 forward?
Tautomerization is a chemical process where a molecule shifts between two forms — in this case, enol-pyruvate to keto-pyruvate.
This change releases energy and stabilizes the product, making the overall conversion from PEP to pyruvate irreversible under physiological conditions and helping ensure ATP is made efficiently.
full process of glycolysis
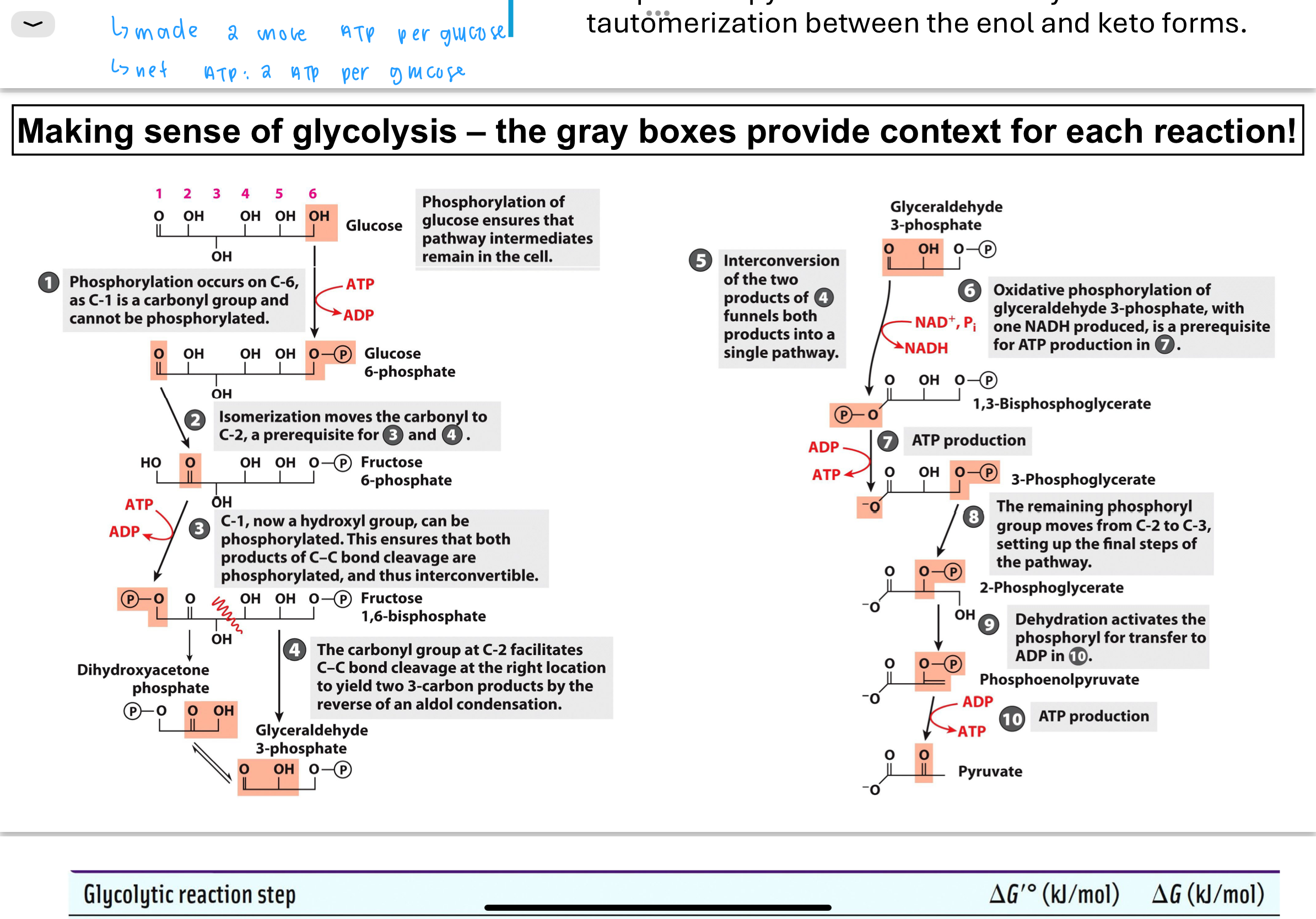
How does the cell drive unfavorable reactions forward in glycolysis?
If a reaction in glycolysis has a positive or close-to-zero ΔGʹ°, the cell can still make it go forward by:
Quickly removing products via subsequent reactions to keep product concentrations low.
Coupling the reaction with another favorable reaction so that the combined ΔGʹ° is negative, making the overall process energetically favorable.
What is the overall net reaction of glycolysis?
Why does fermentation happen?
fermentation occurs when there is no/low oxygen levels
it’s main job is to make NAD+ from NADH so glycolysis can keep going
it converts pyruvate into lactic acid (in humans) or ethanol and CO2 (yeast).
this keeps ATP production going under anaerobic conditions
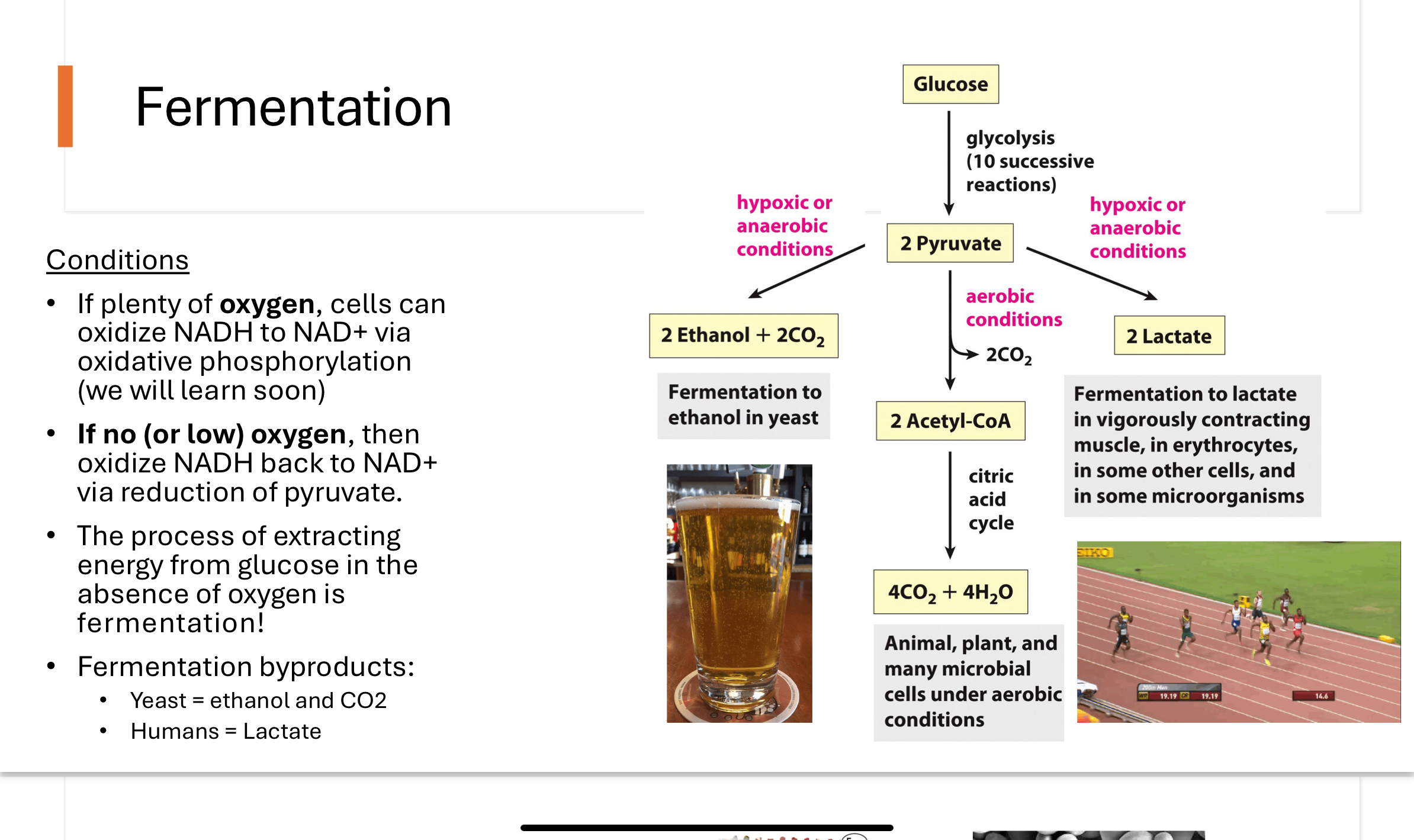
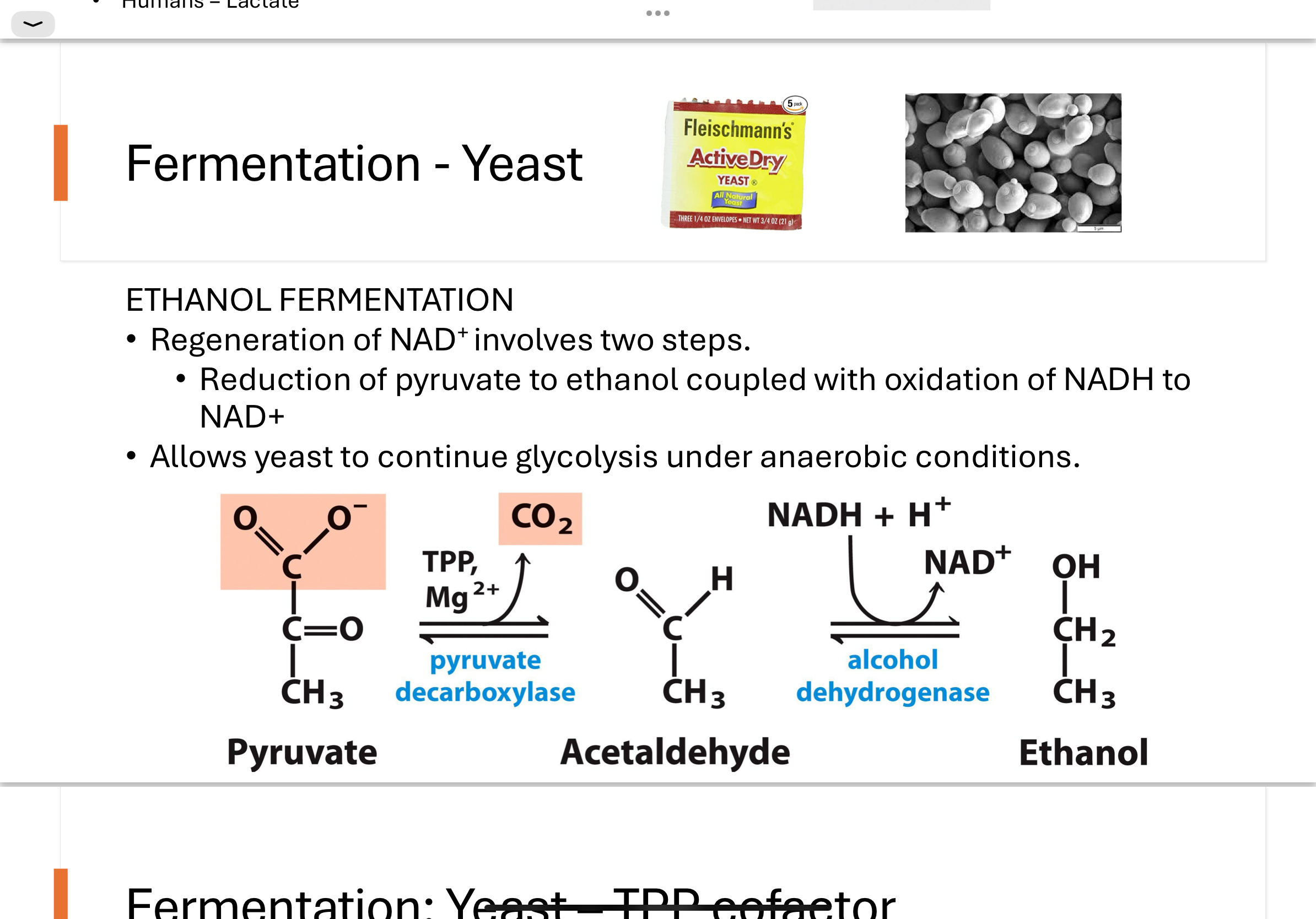
How does ethanol fermentation work in yeast?
Ethanol fermentation occurs in two steps:
Pyruvate is decarboxylated to acetaldehyde by the enzyme pyruvate decarboxylase (humans do not have this enzyme).
Acetaldehyde is reduced to ethanol, while NADH is oxidized to NAD⁺ by the enzyme alcohol dehydrogenase.
This regeneration of NAD⁺ allows glycolysis to continue in anaerobic conditions.
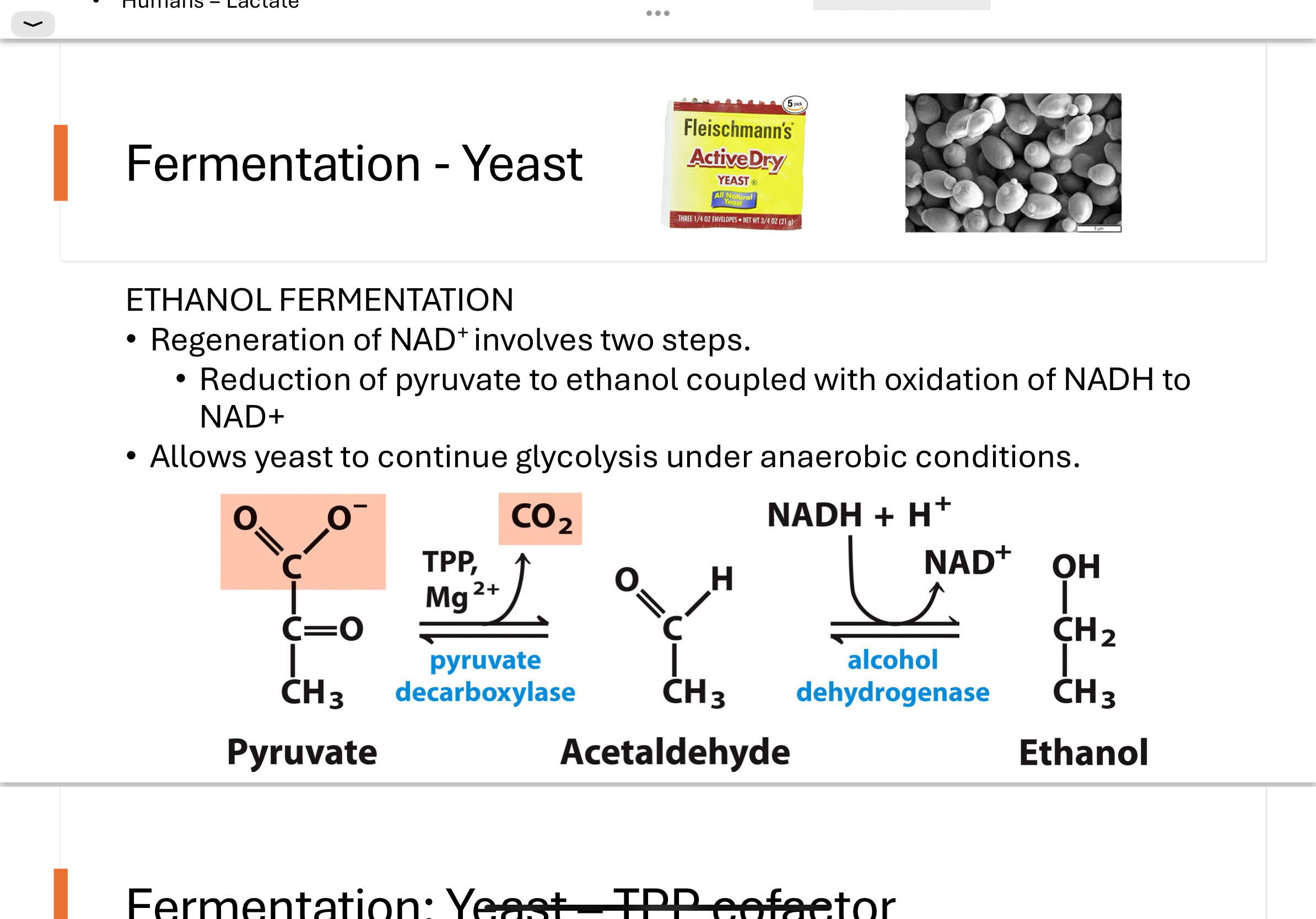
fermentation yeast— TPP cofactor
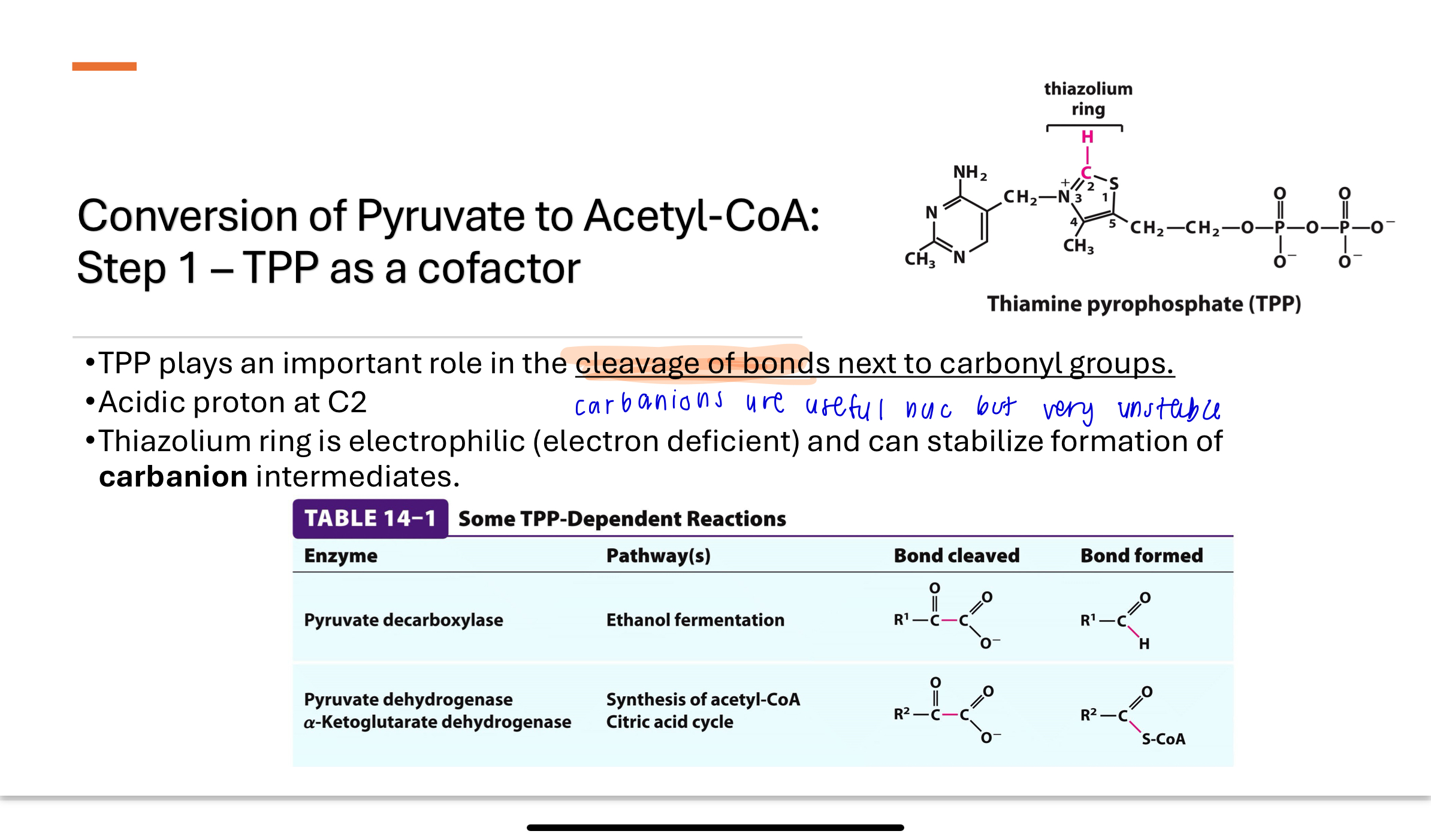
-Thiamine Pyrophosphate (TPP) plays an important role in the cleavage of bonds next to carbonyl groups.
Thiazolium ring can deprotonate to form a highly reactive carbanion (pink C-H)
Thiazolium ring acts as an electron acceptor to facilitate reactions
Know mechanism for ethanol fermentation step 1— yeast
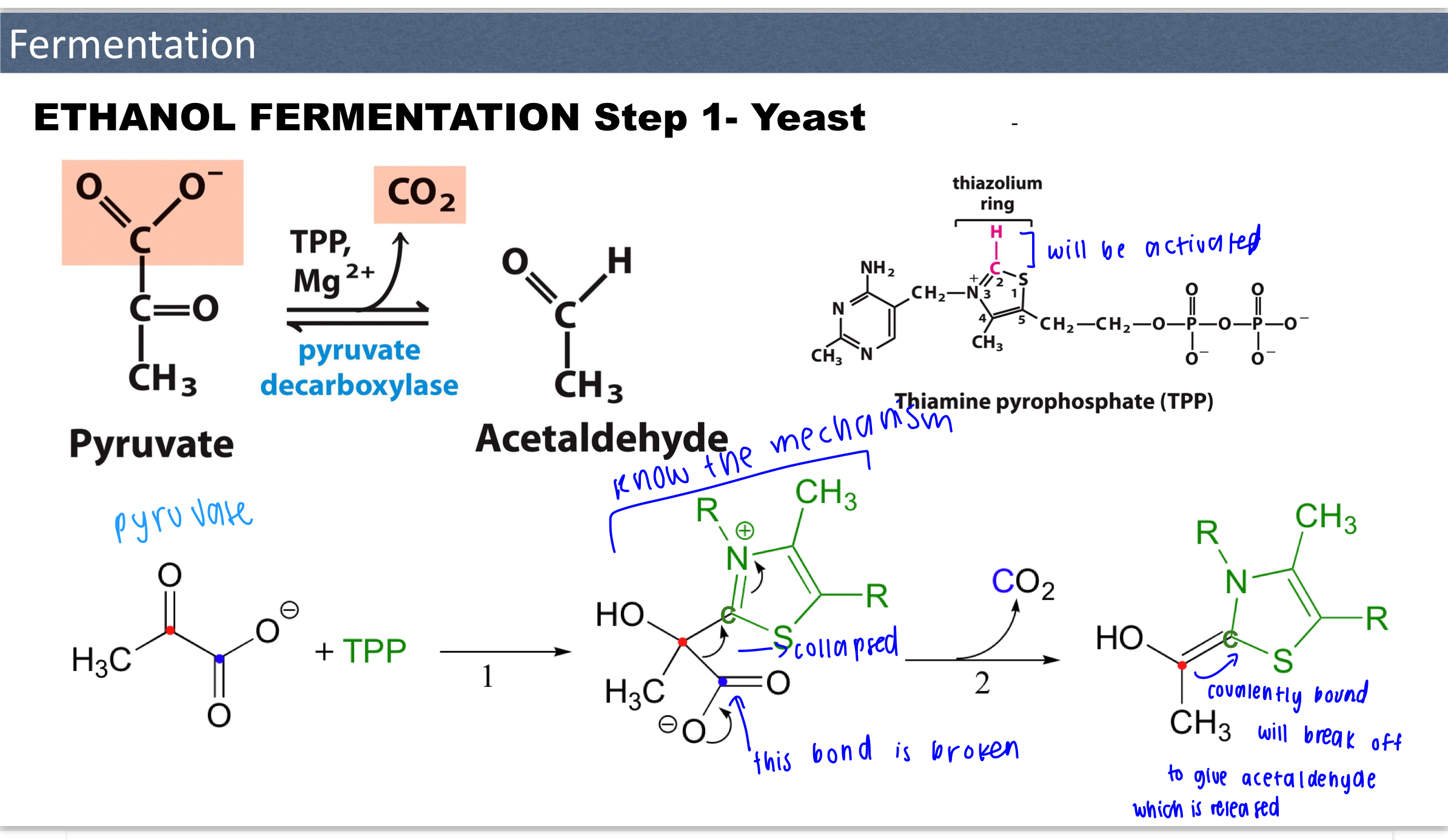
Step 2 for ethanol fermentation (yeast)
Acetaldehyde is reduced to EtOH and NADH is oxidized to NAD+
Humans lack the gene for pyruvate decarboxylase, so they cannot convert pyruvate to ethanol. However, humans do have alcohol dehydrogenase, which works in reverse: in the liver, it oxidizes ethanol to acetaldehyde, a step in alcohol metabolism rather than fermentation.
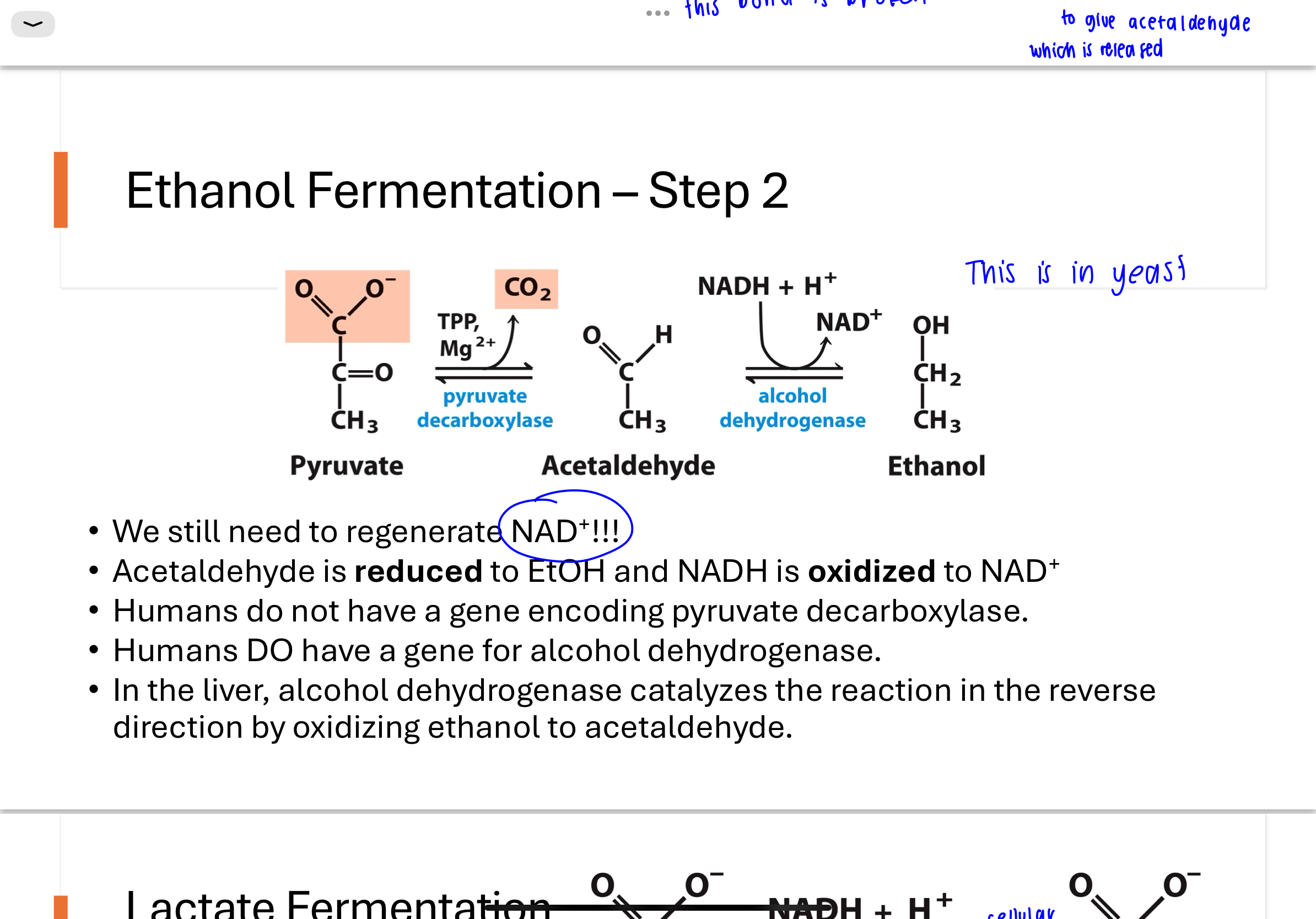
lactate fermentation - humans
During intense exercise, oxygen supply to muscle cells is insufficient. To regenerate NAD⁺, cells reduce pyruvate to lactate using lactate dehydrogenase, while oxidizing NADH to NAD⁺. This allows glycolysis to continue producing ATP anaerobically, though it results in the buildup of lactic acid.
Q: What is gluconeogenesis, and why is it important?
Gluconeogenesis is the biosynthetic pathway that creates glucose from non-carbohydrate sources such as lactate, pyruvate, some amino acids, and glycerol.
It’s especially important in red blood cells, brain, and embryonic tissues, which rely heavily on glucose.
In humans, gluconeogenesis is essential during fasting, starvation, or extended exercise to maintain blood glucose levels.
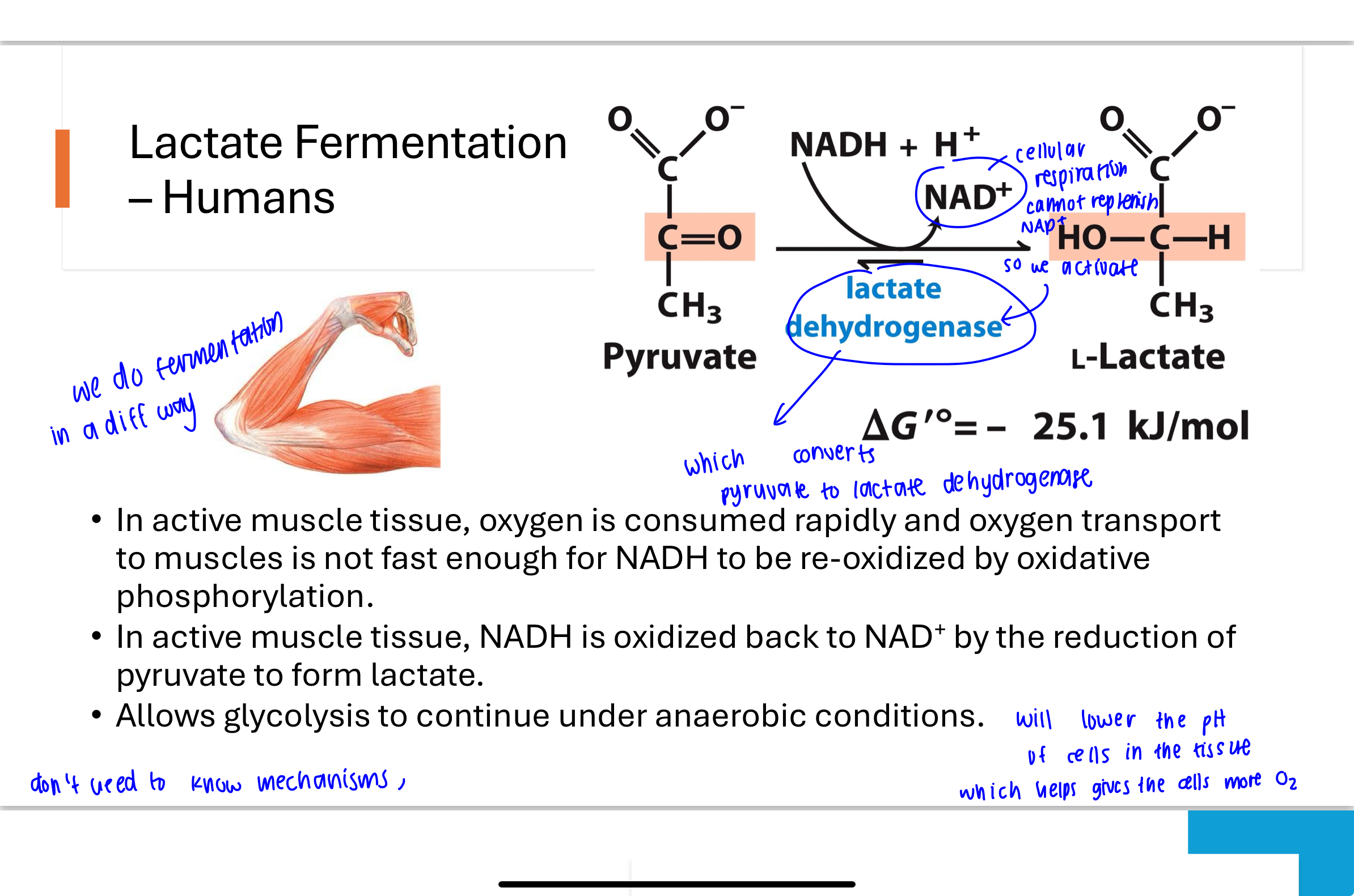
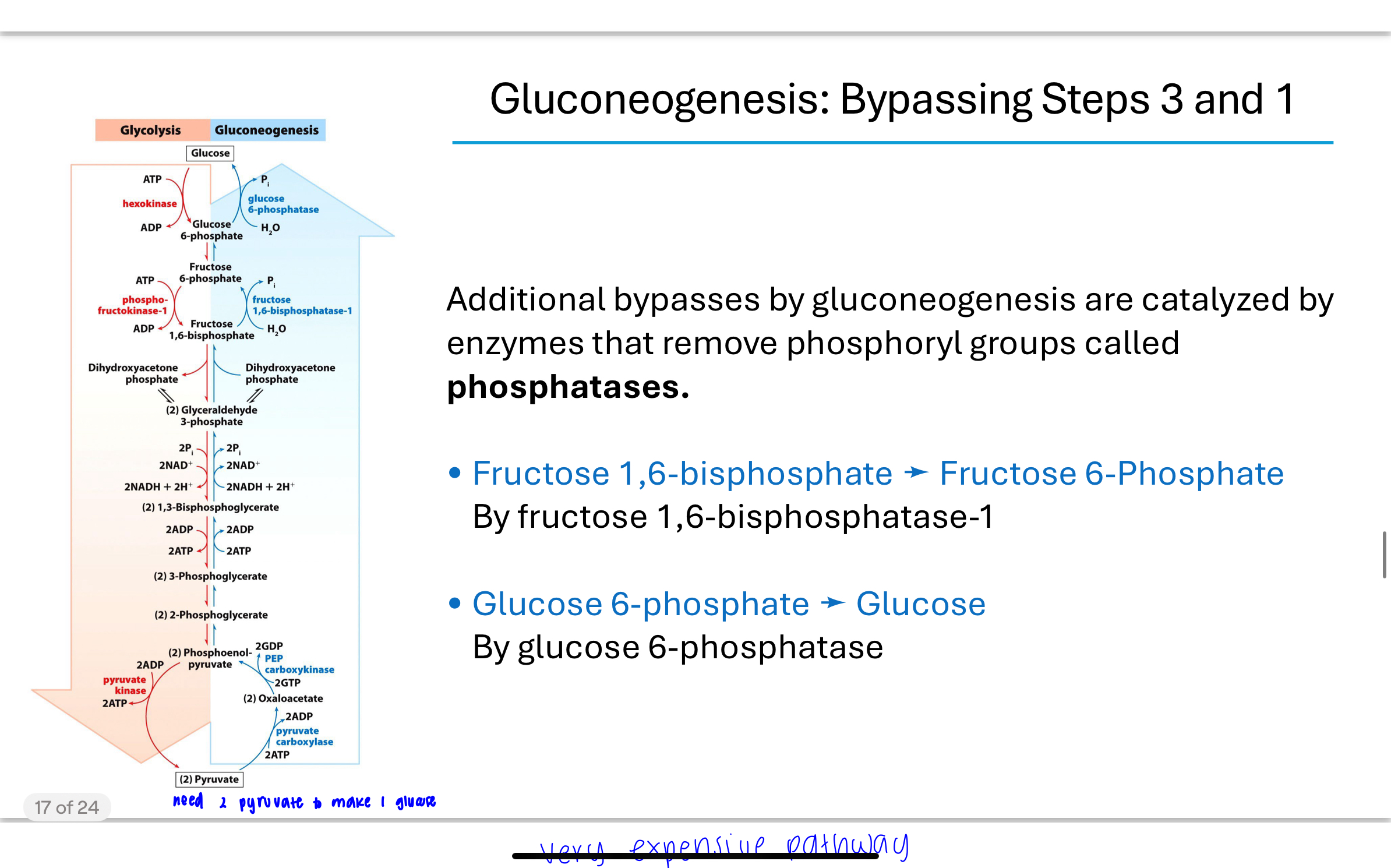
Q: Which steps of glycolysis must be bypassed during gluconeogenesis and why?
Most glycolysis steps are reversible, but steps 1, 3, and 10 are irreversible due to their large negative ΔG values. These steps must be bypassed by alternative reactions with different enzymes:
Step 1: Glucose 6-phosphatase
Step 3: Fructose 1,6-bisphosphatase
Step 10: Pyruvate carboxylase and PEP carboxykinase
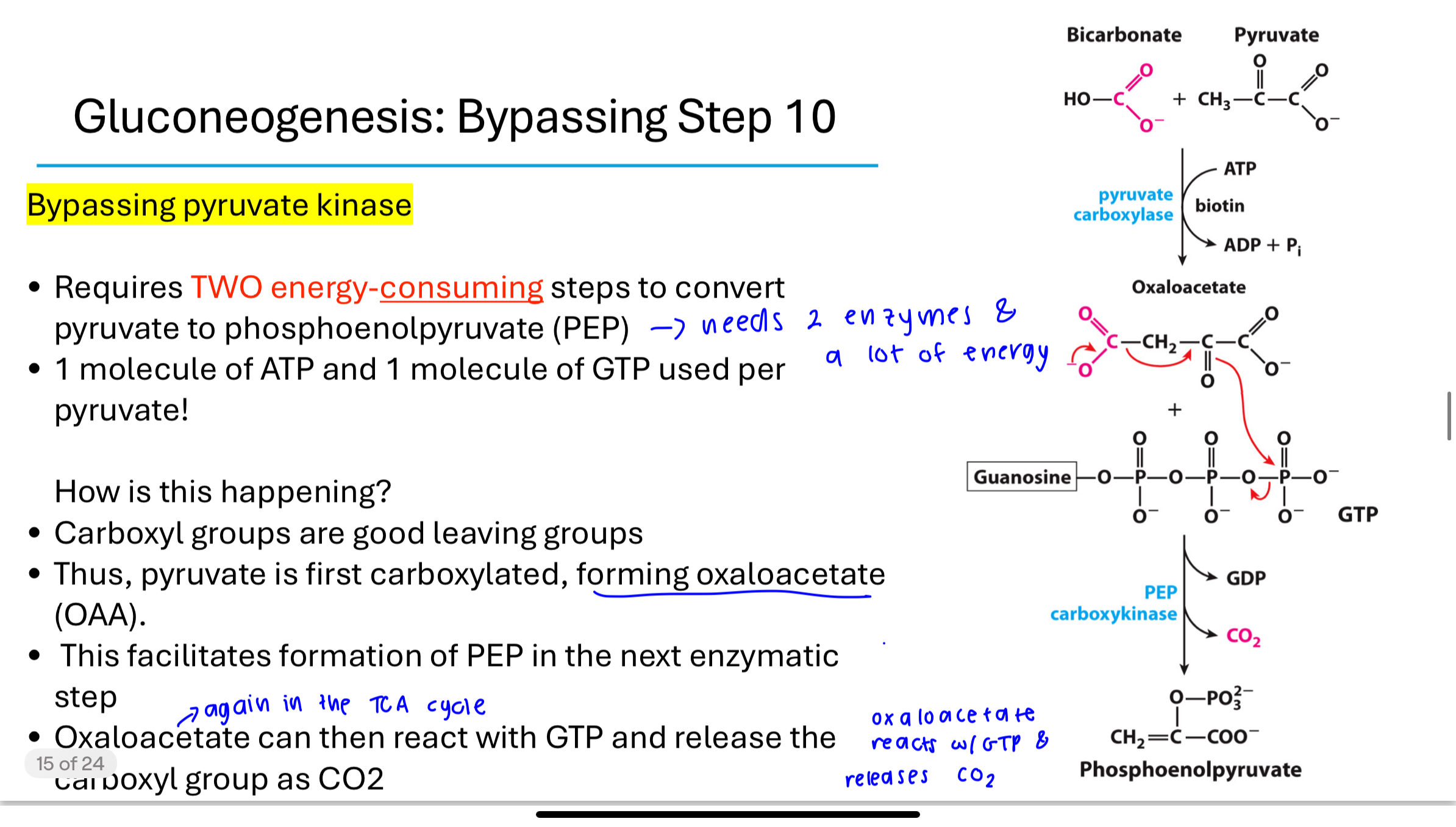
gluconeogenesis is energetically expensive
To synthesize one molecule of glucose, gluconeogenesis consumes:
4 ATP
2 GTP
2 NADH
This is energetically expensive but necessary for survival during glucose scarcity. Glycolysis and gluconeogenesis are reciprocally regulated to prevent both from occurring simultaneously.but it is necessary sometimes like during fasting, starvation or exercise,
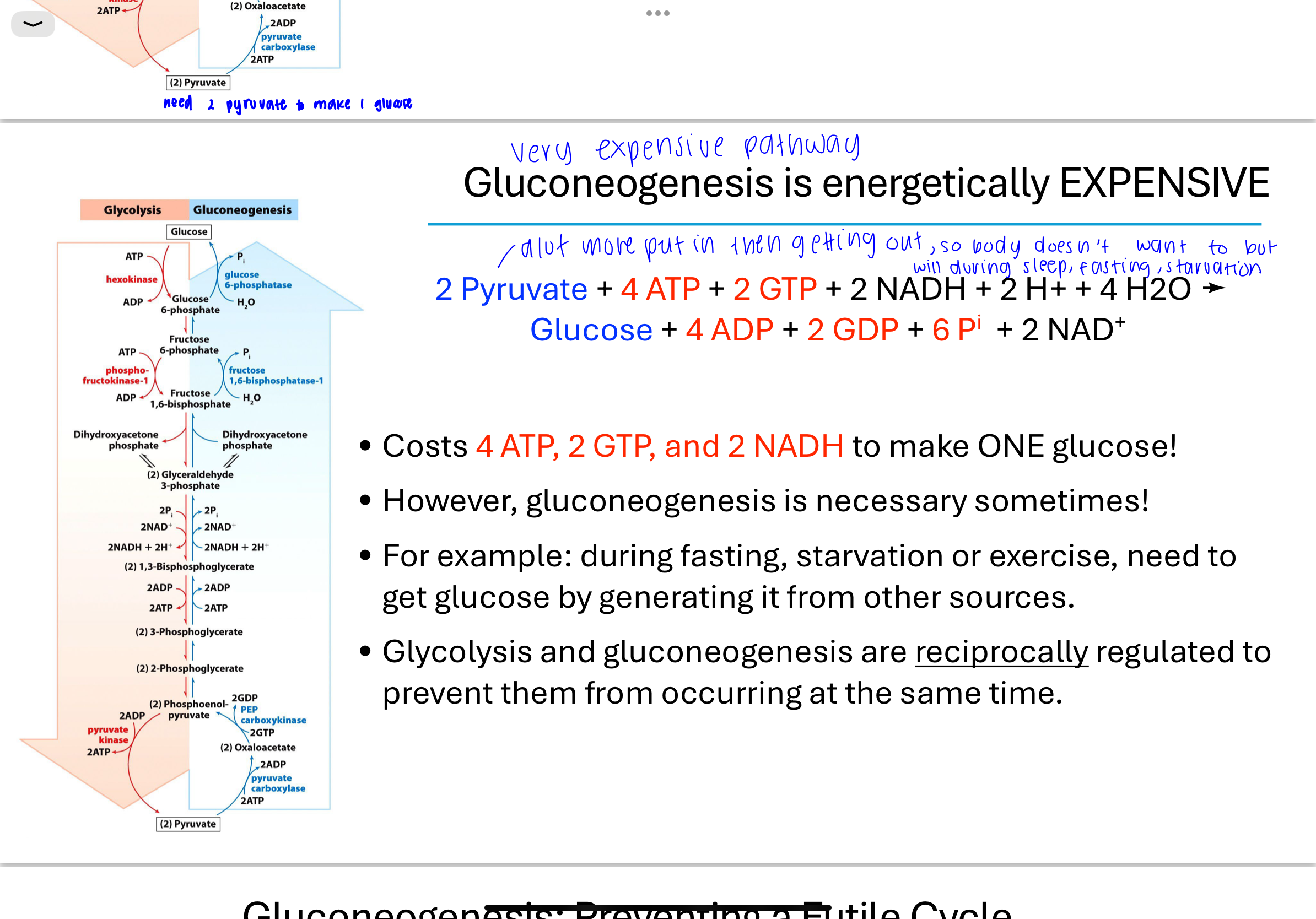
Q: Where does gluconeogenesis primarily occur in the body?
While glycolysis occurs in almost all cells, gluconeogenesis is mainly carried out in the liver (and to a lesser extent in the kidneys). This tissue-specific separation helps avoid futile cycles where glucose would be simultaneously broken down and synthesized.
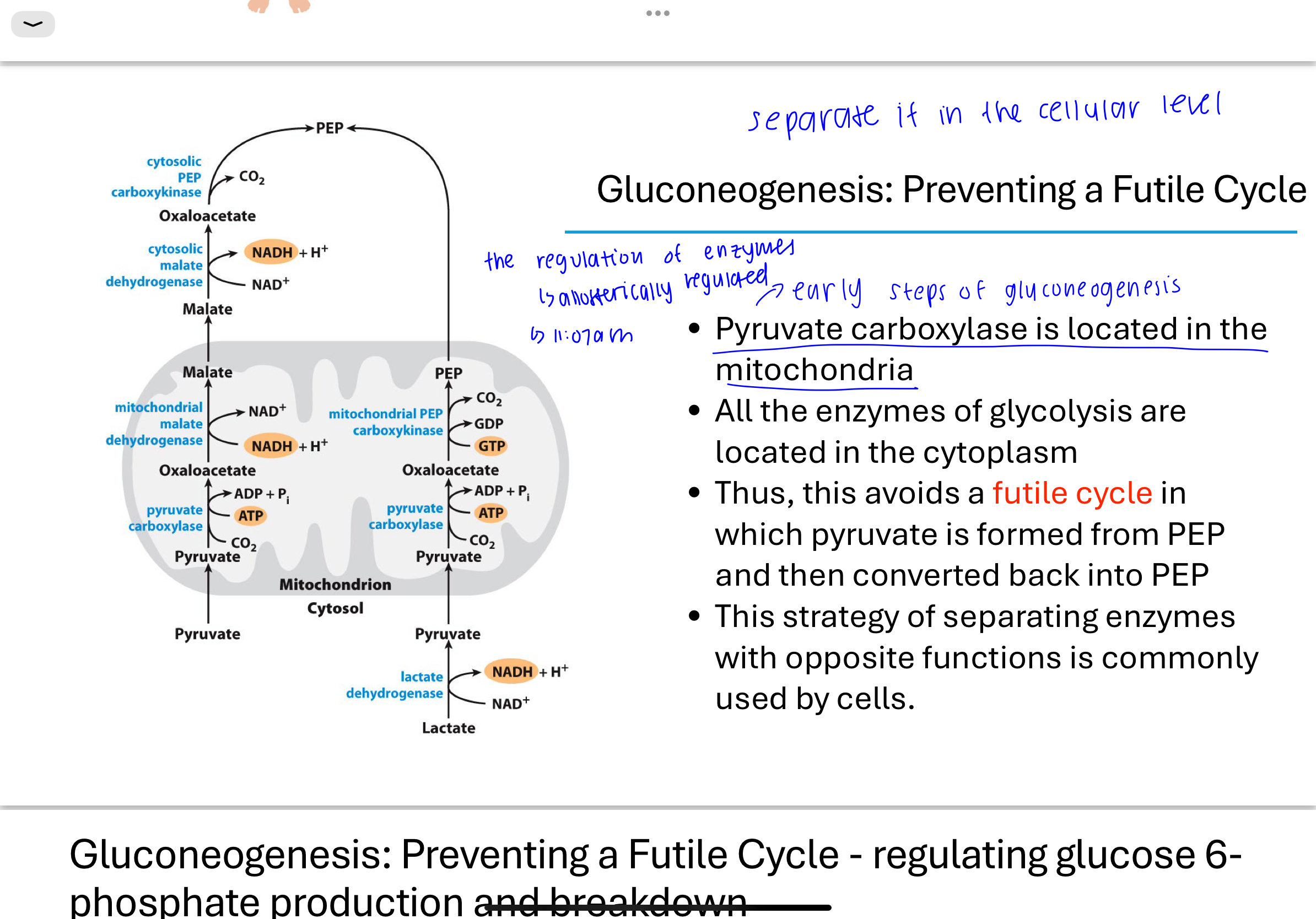
Q: How do cells prevent a futile cycle between PEP and pyruvate?
Cells localize pyruvate carboxylase to the mitochondria, while glycolysis enzymes like pyruvate kinase are in the cytoplasm.
This physical separation of enzymes prevents the simultaneous conversion of pyruvate to PEP and back again, which would waste energy.
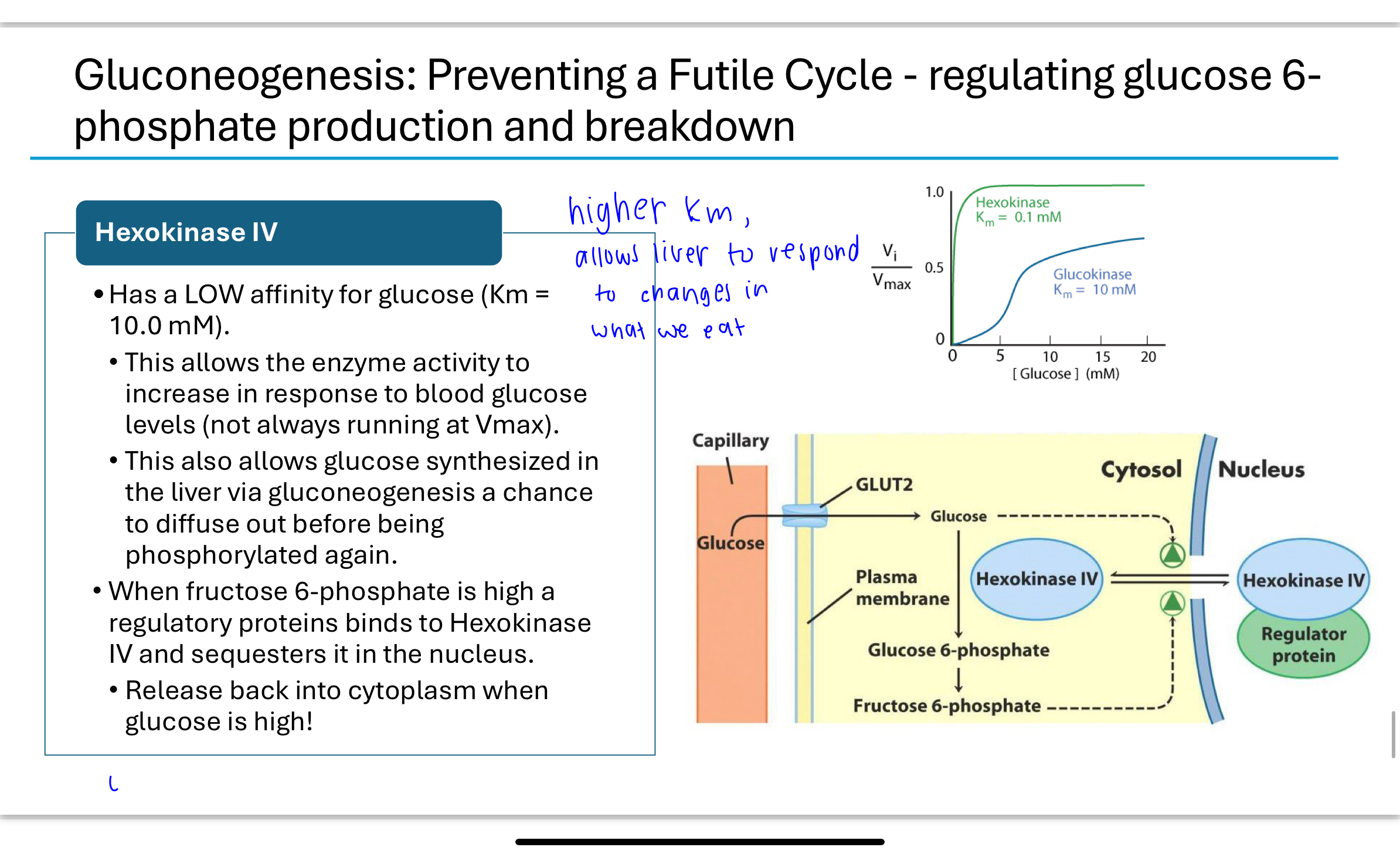
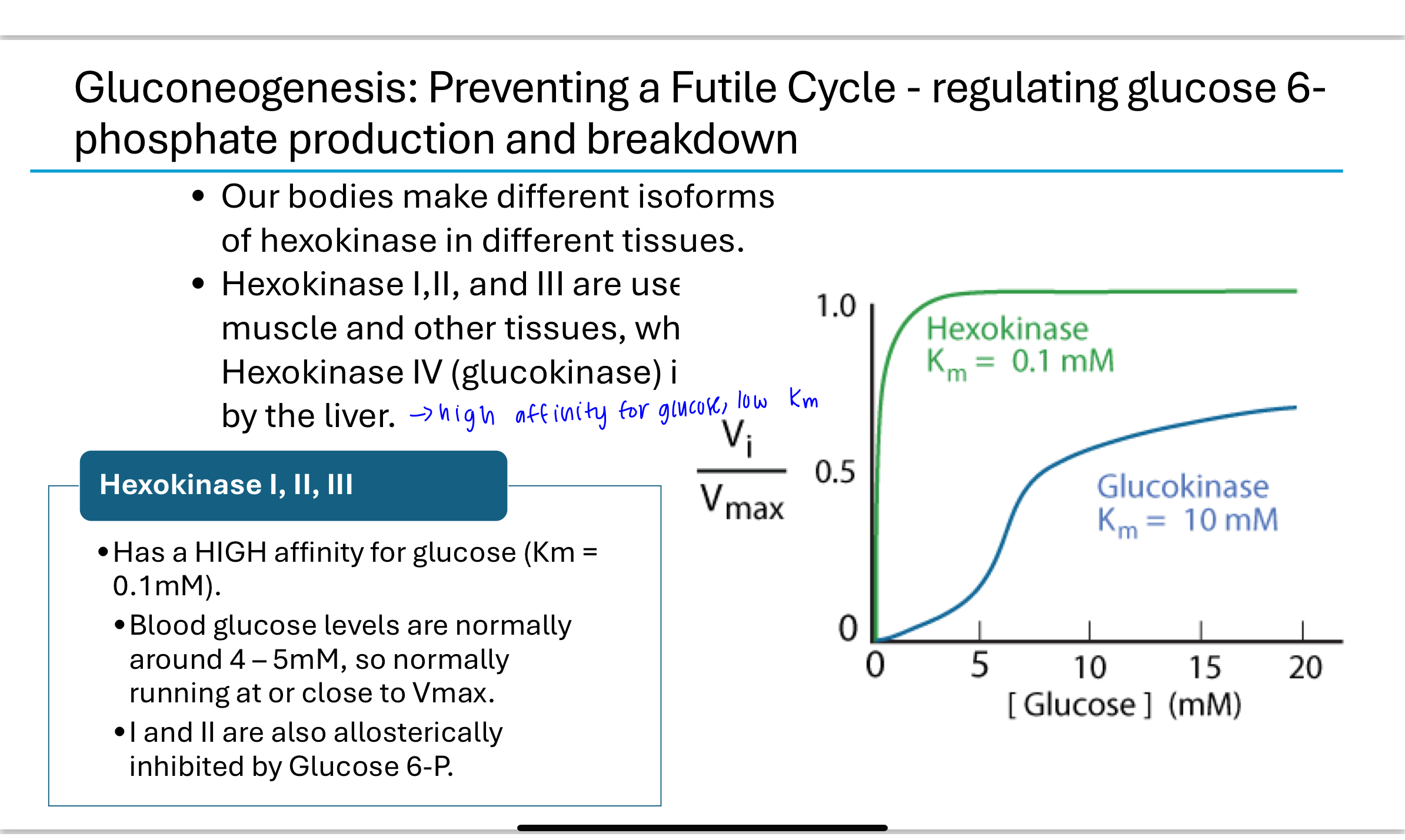
What role do hexokinase isoforms play in regulating glucose metabolism?
Different tissues express different hexokinase isoforms:
Hexokinase I, II, III: Found in muscle and most tissues; high affinity for glucose (low Km), always active at physiological glucose levels.
Hexokinase IV (glucokinase): Found in the liver; low affinity (high Km), allowing glucose to leave the liver when blood glucose is low and be phosphorylated only when glucose levels are high. Glucokinase is also regulated by sequestration in the nucleus when fructose 6-phosphate levels are high.
What regulates entry into the pentose phosphate pathway?
The PPP is regulated by NADPH levels. When NADPH is high, it inhibits glucose-6-phosphate dehydrogenase, the enzyme that catalyzes the first step of the pathway. This ensures that glucose-6-phosphate is redirected back to glycolysis when NADPH supply is sufficient.
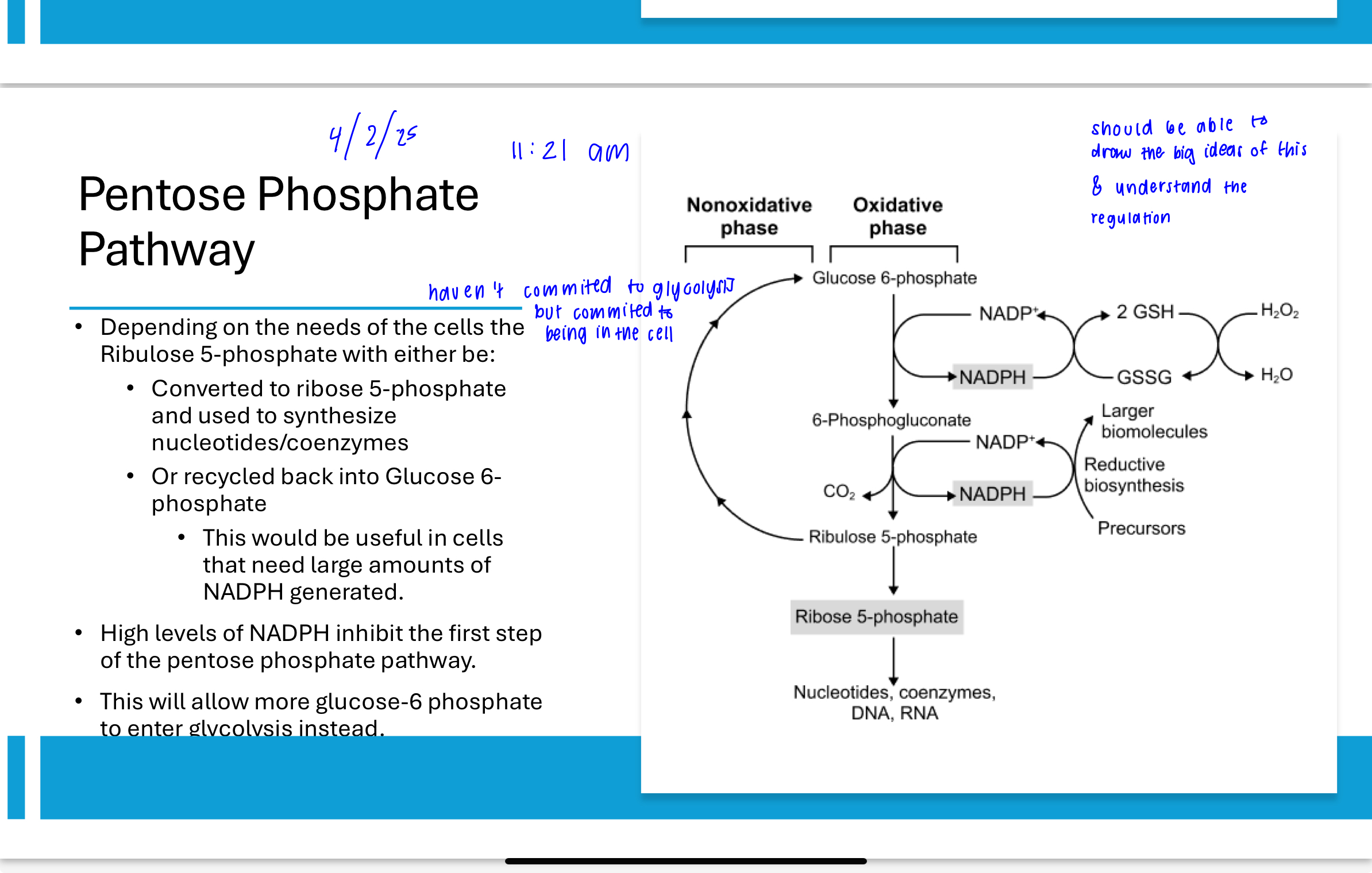
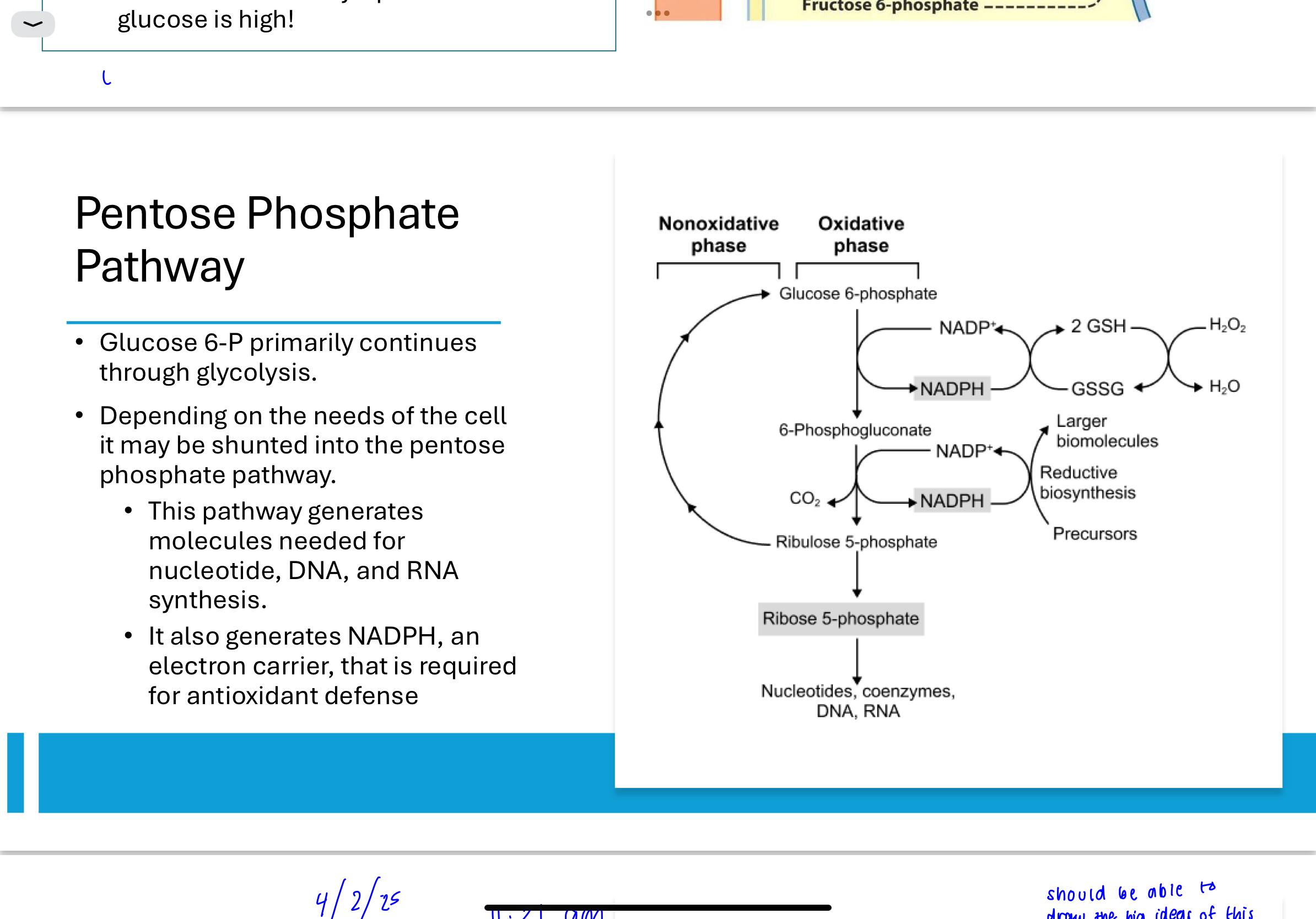
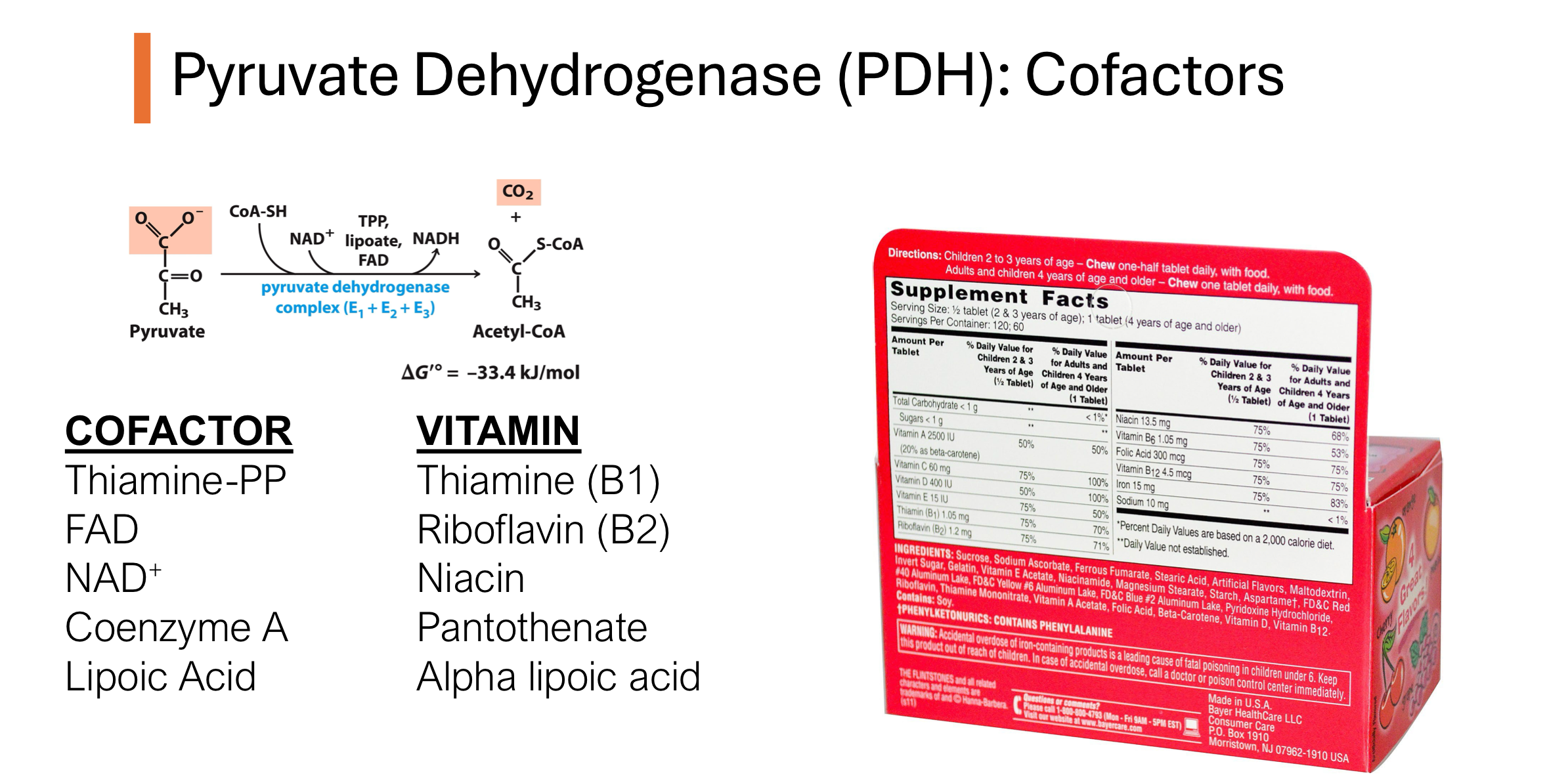
How does pyruvate dehydrogenase relate to glycolysis
Pyruvate dehydrogenase (PDH) is a multi-enzyme complex that plays a critical role in cellular respiration.
Its main function is to convert pyruvate, the end product of glycolysis, into acetyl-CoA, a key molecule that enters the citric acid cycle (TCA or Krebs cycle) for further energy production.
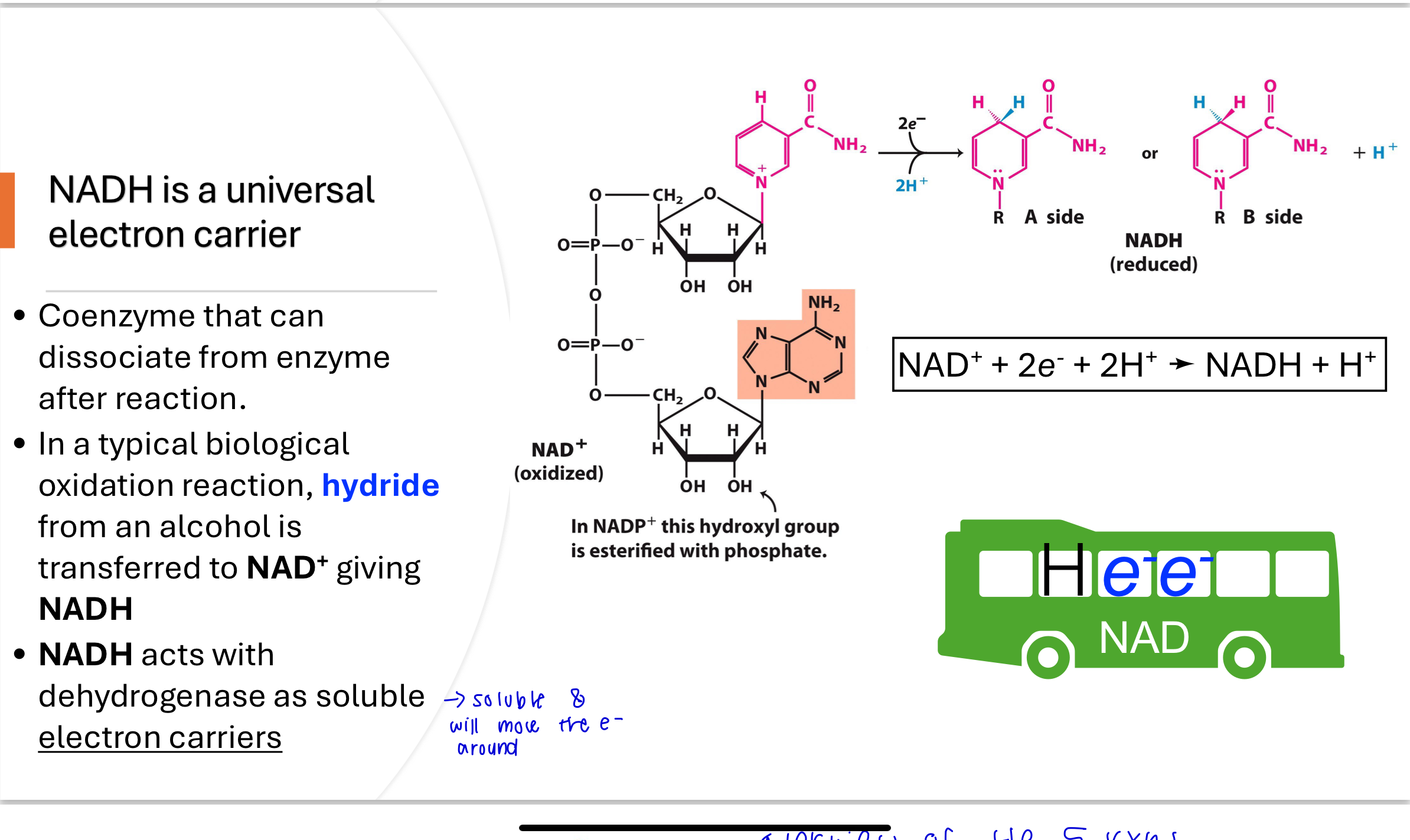
Q: What is the pyruvate dehydrogenase reaction, and where does it occur?
The PDH complex catalyzes the oxidative decarboxylation of pyruvate to acetyl-CoA. This multi-subunit enzyme (E1, E2, E3) is located in the mitochondrial matrix and is highly thermodynamically favorable. It is the first point where carbons from glucose are fully oxidized to CO₂.
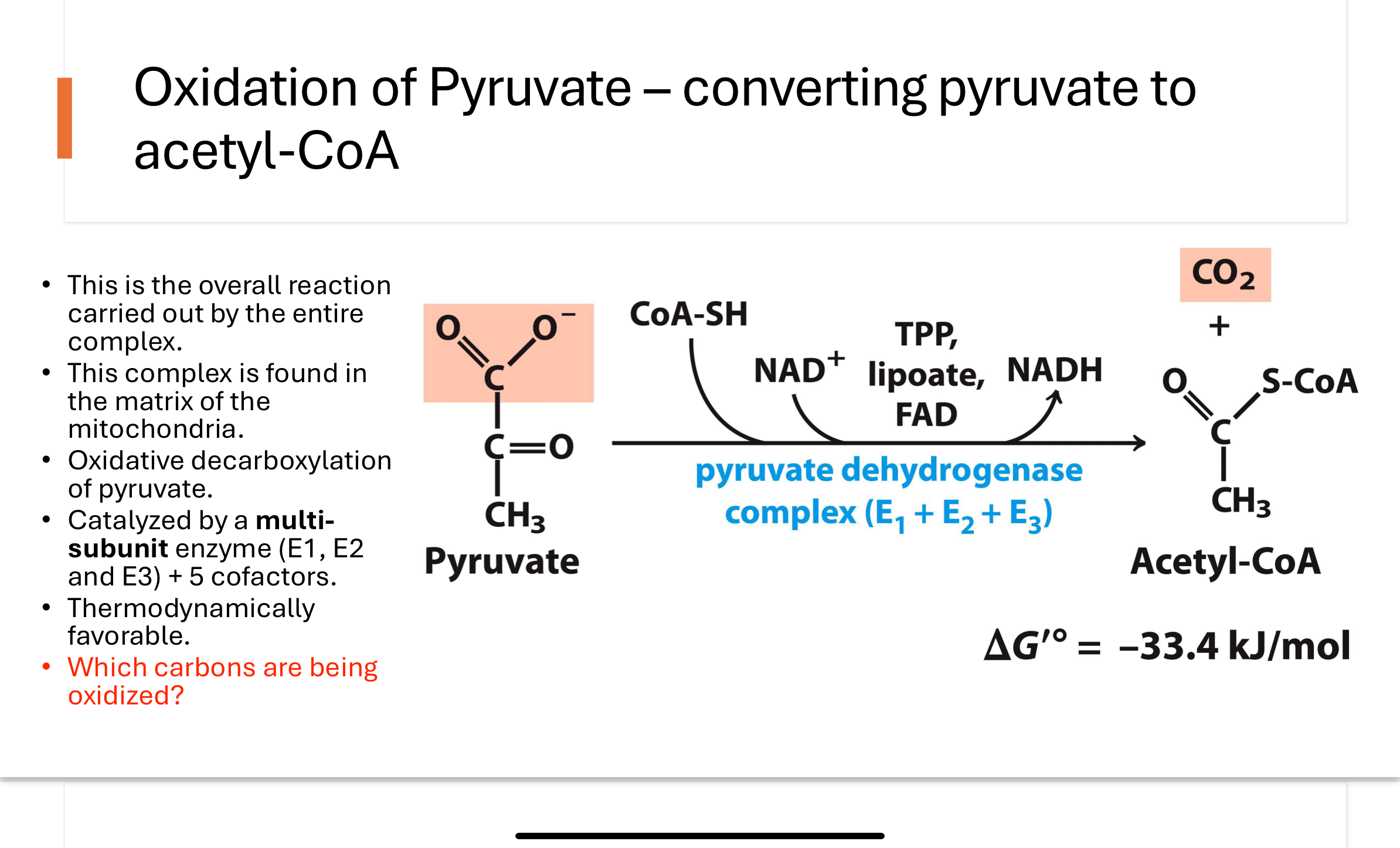
Q: What are the components of the pyruvate dehydrogenase complex?
The PDH complex includes:
E1: Pyruvate dehydrogenase (uses TPP)
E2: Dihydrolipoamide transacetylase (uses lipoic acid & CoA)
E3: Dihydrolipoamide dehydrogenase (uses FAD & NAD⁺)
These enzymes work together with five cofactors to convert pyruvate into acetyl-CoA and CO₂.
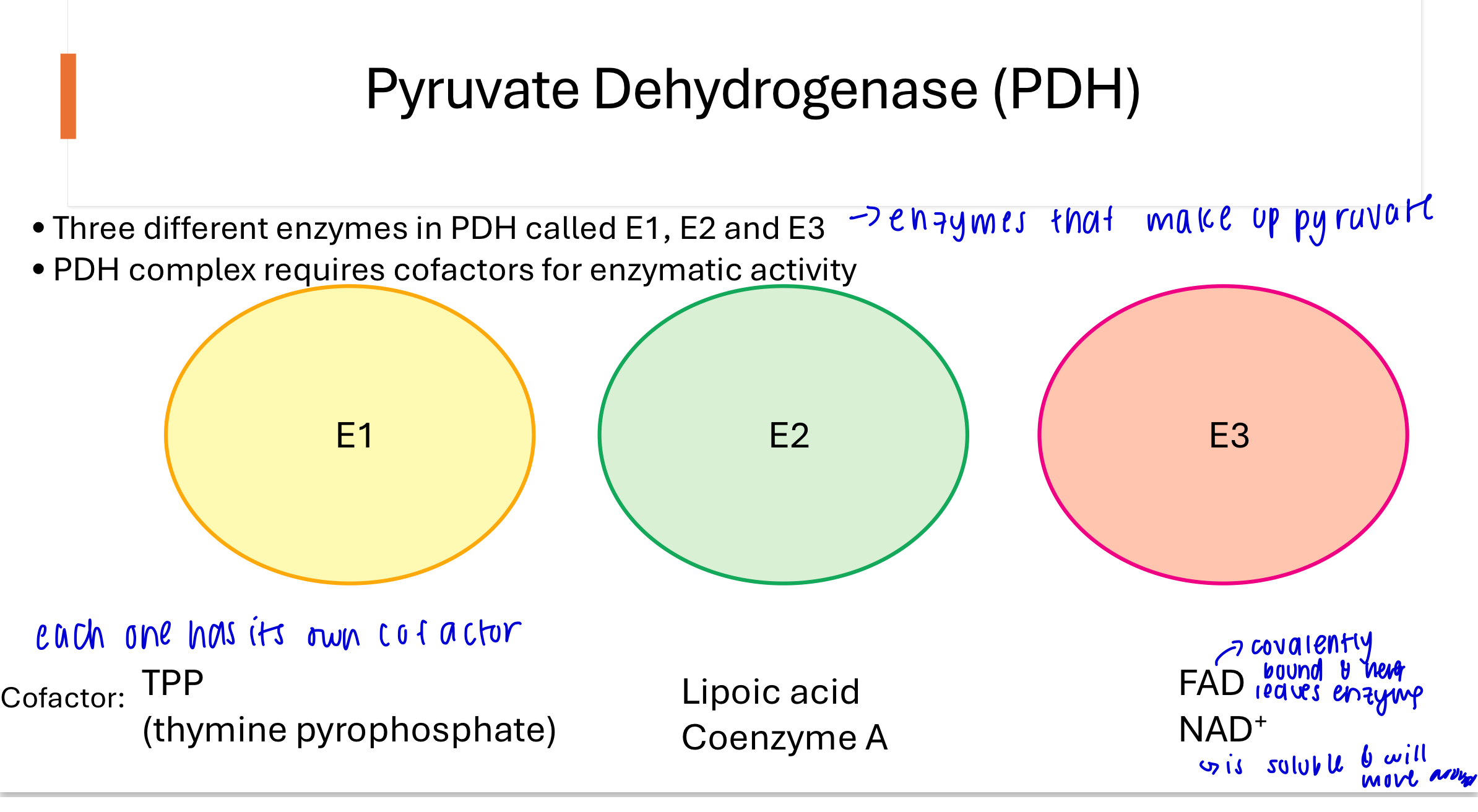
pyruvate dehydrogenase cofactors
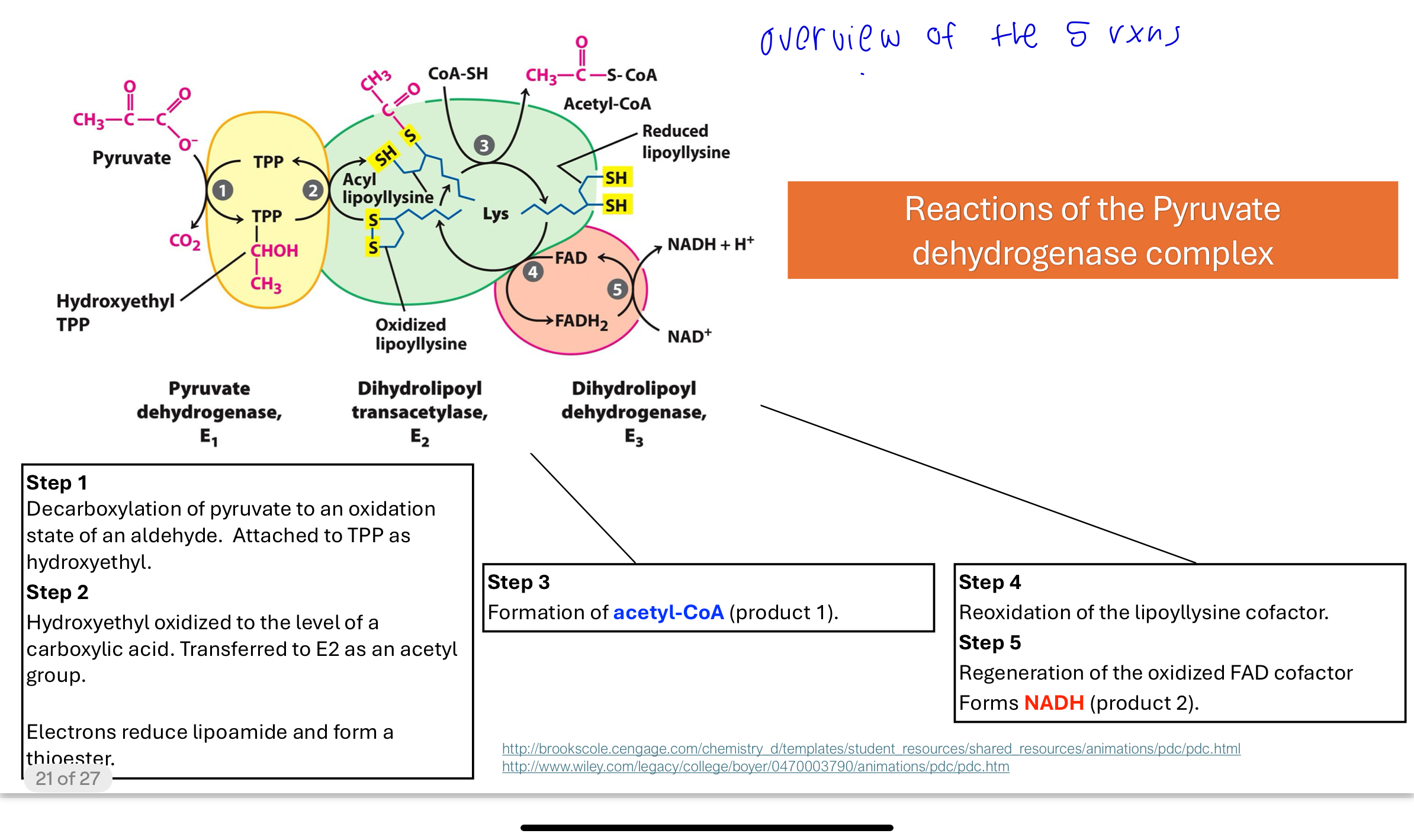
What happens in Step 1 of pyruvate conversion to acetyl-CoA?
In Step 1:
E1 catalyzes decarboxylation of pyruvate using TPP.
Pyruvate loses a CO₂, and the remaining 2-carbon unit forms a hydroxyethyl-TPP intermediate.
This carbon is now in an oxidized aldehyde state.
This step mirrors pyruvate decarboxylase in yeast fermentation.
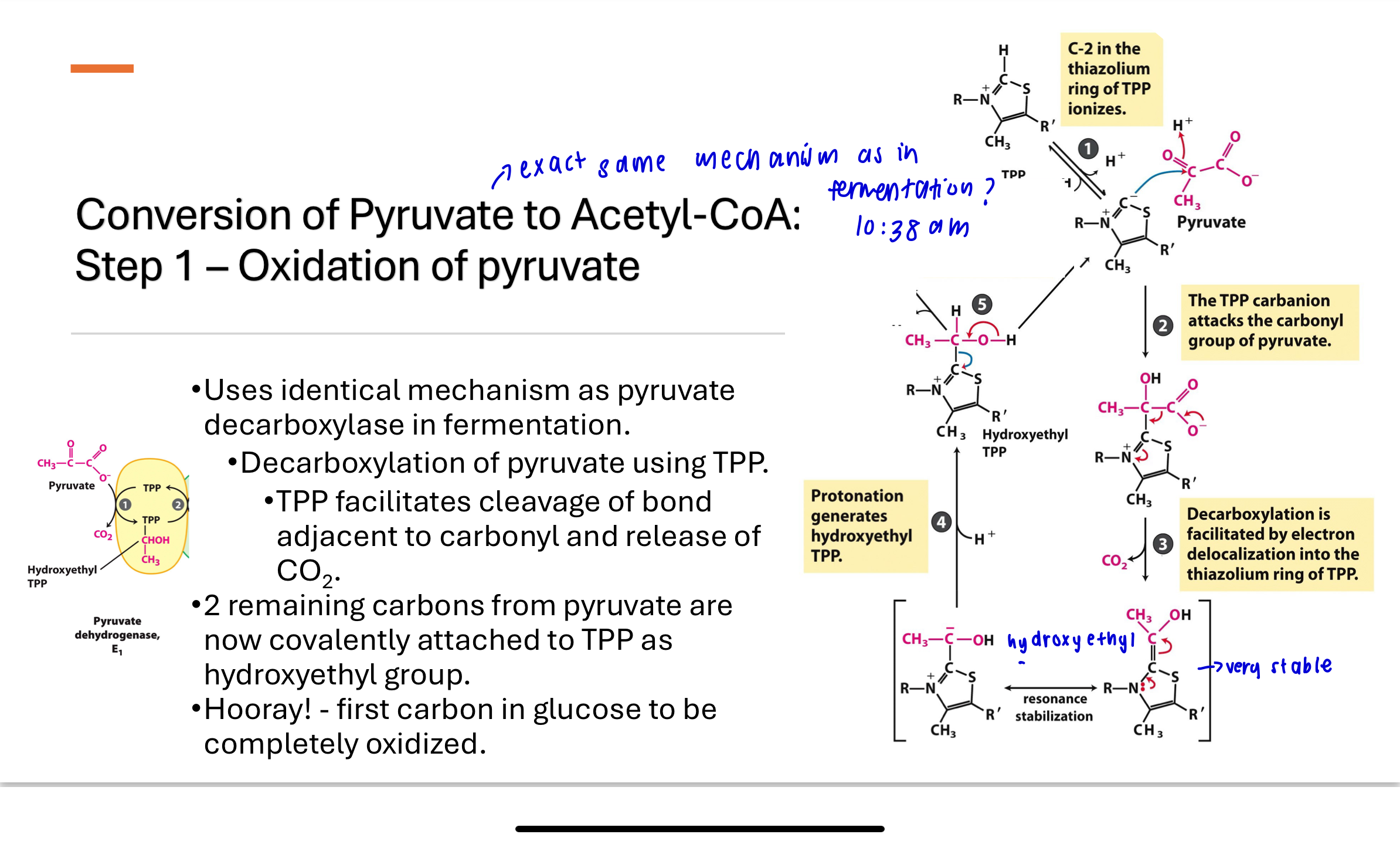
How does TPP function as a cofactor in E1?
Uses TPP as a cofactor which plays an important role in the cleavage of bonds next to carbonyl groups
acidic proton at C2
The thiazolium ring is electrophilic (electron deficient) and can stabilize the formation of carbanion intermediates
conversion of Pyruvate to Acetyl-CoA step 2—Generating Acetyl-CoA
Lipollysine is a prosthetic group
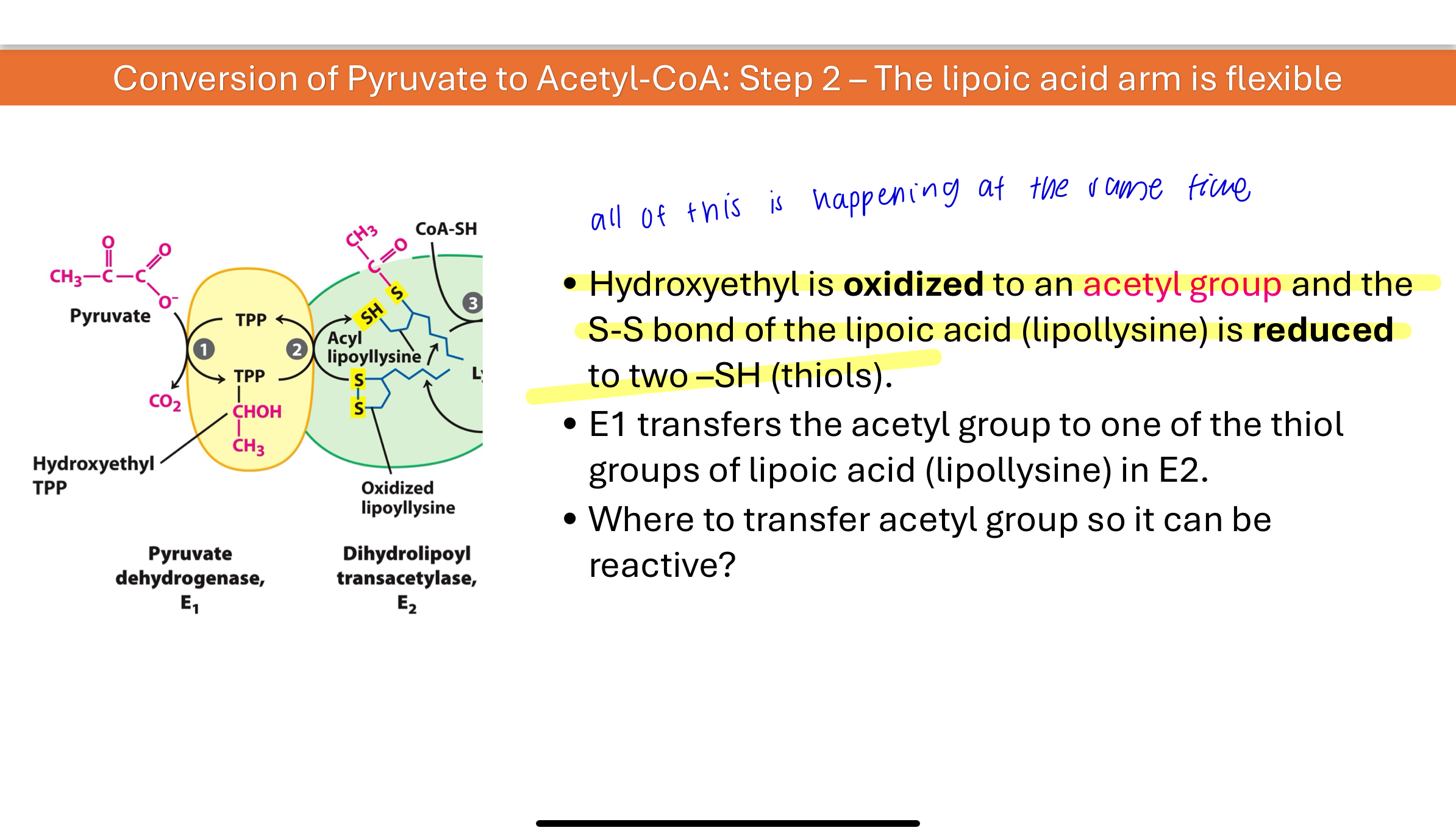
The hydroxyethyl group is oxidized to an acetyl group, and electrons reduce lipoic acid (lipoyllysine) on E2. The acetyl group is transferred to one of the thiol groups of lipoic acid, forming a high-energy thioester intermediate.
The lipoic acid acts as both an electron and acyl carrier.
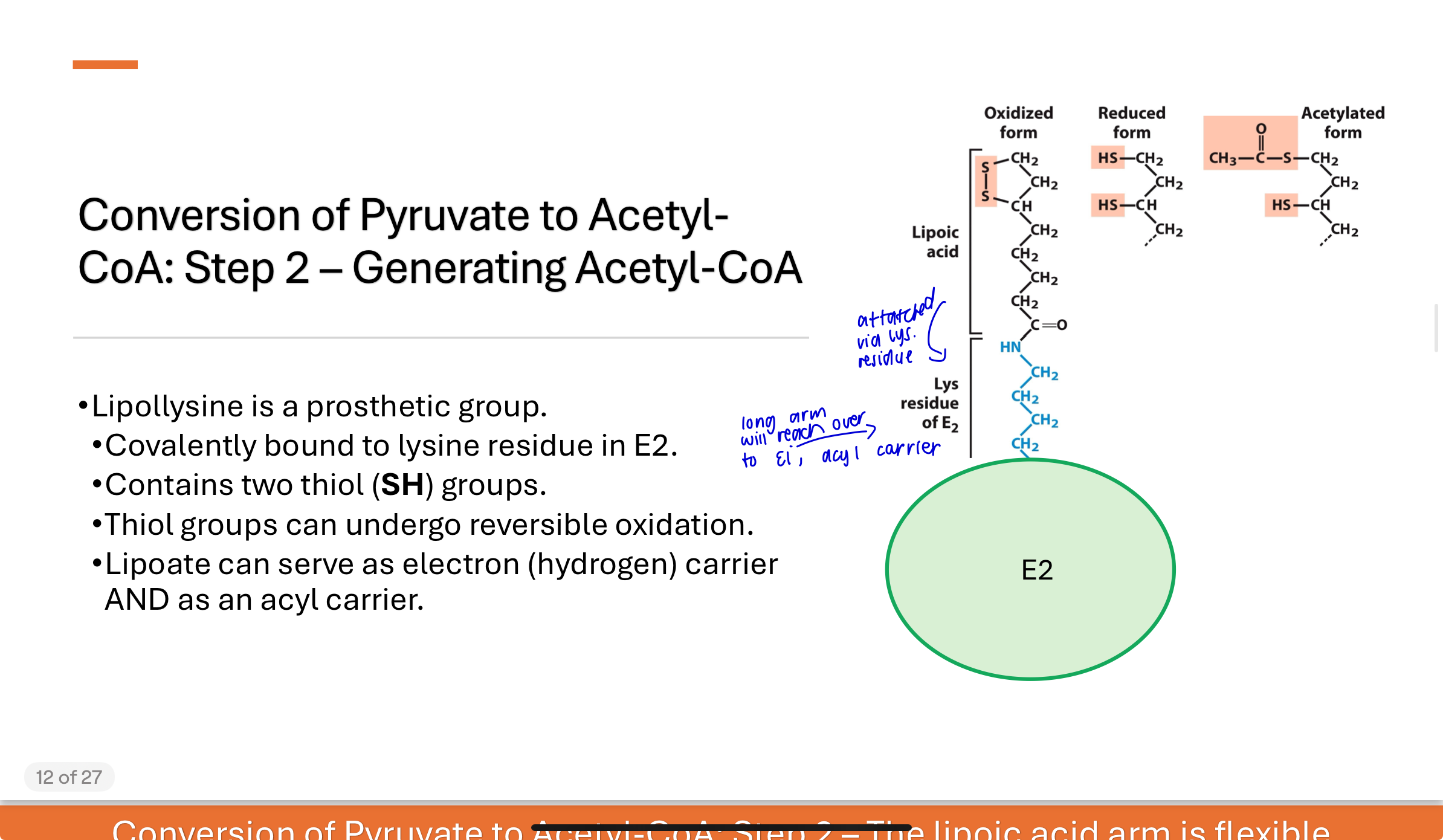
Q: What is the structure and function of the lipoic acid “arm”?
Lipoic acid is covalently attached to a lysine side chain in E2, forming lipoyllysine. This arm:
Contains two thiol (–SH) groups that can be reversibly oxidized/reduced
Acts as a swinging arm to carry intermediates between enzyme active sites
Enables efficient substrate channeling from E1 → E2 → E3
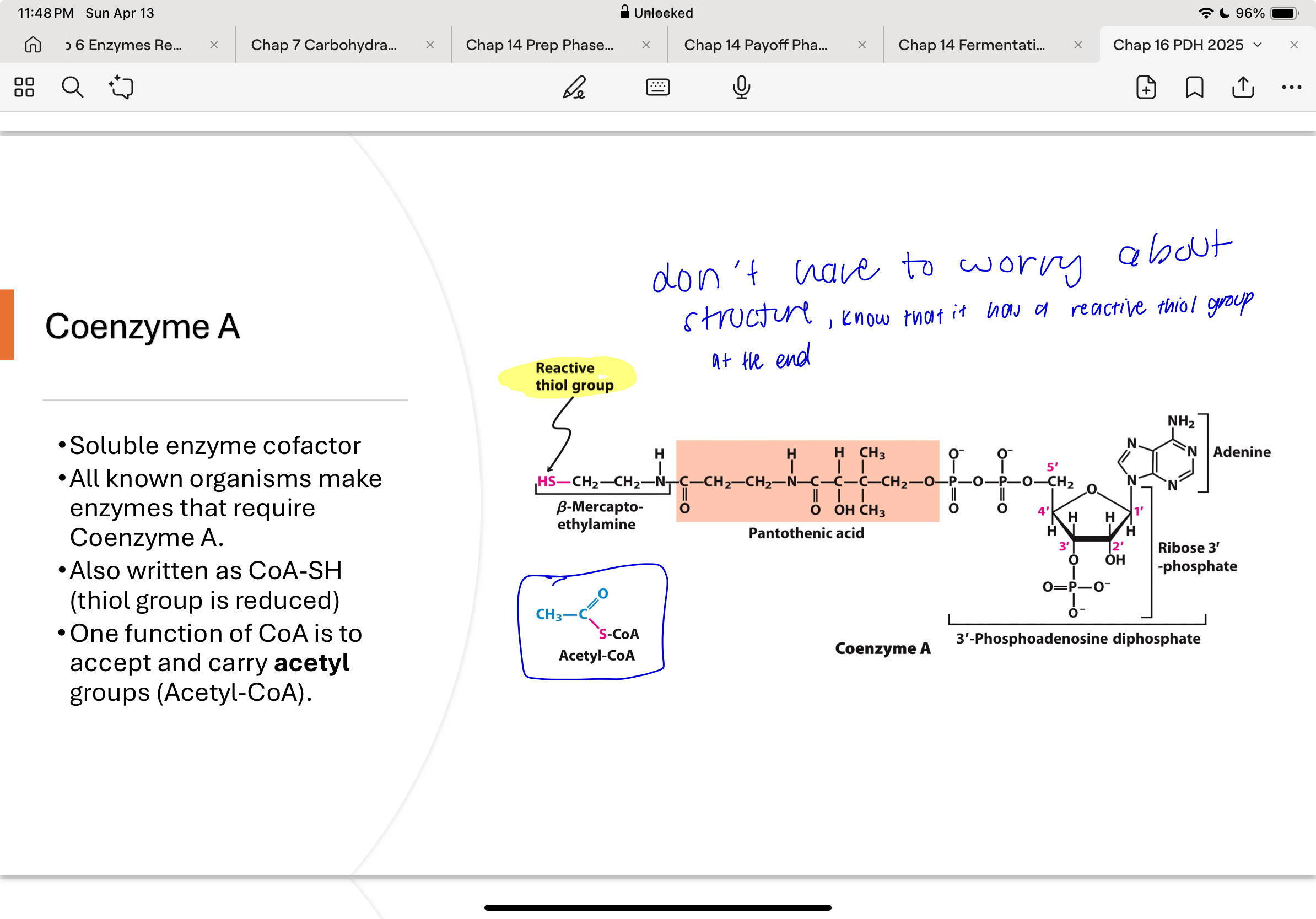
coenzyme A
Coenzyme A:
Accepts the acetyl group from reduced lipoyllysine
Forms acetyl-CoA, a thioester with high energy potential
Is a soluble cofactor used in multiple metabolic pathways
Allows transfer of acetyl groups for use in the citric acid cycle
Q: What happens in Step 3 of the PDH complex reaction?
The acetyl group is transferred from lipoic acid to CoA-SH, forming acetyl-CoA. This produces the first product of the complex, but the lipoic acid now has reduced thiol groups that need to be reoxidized in the next step.
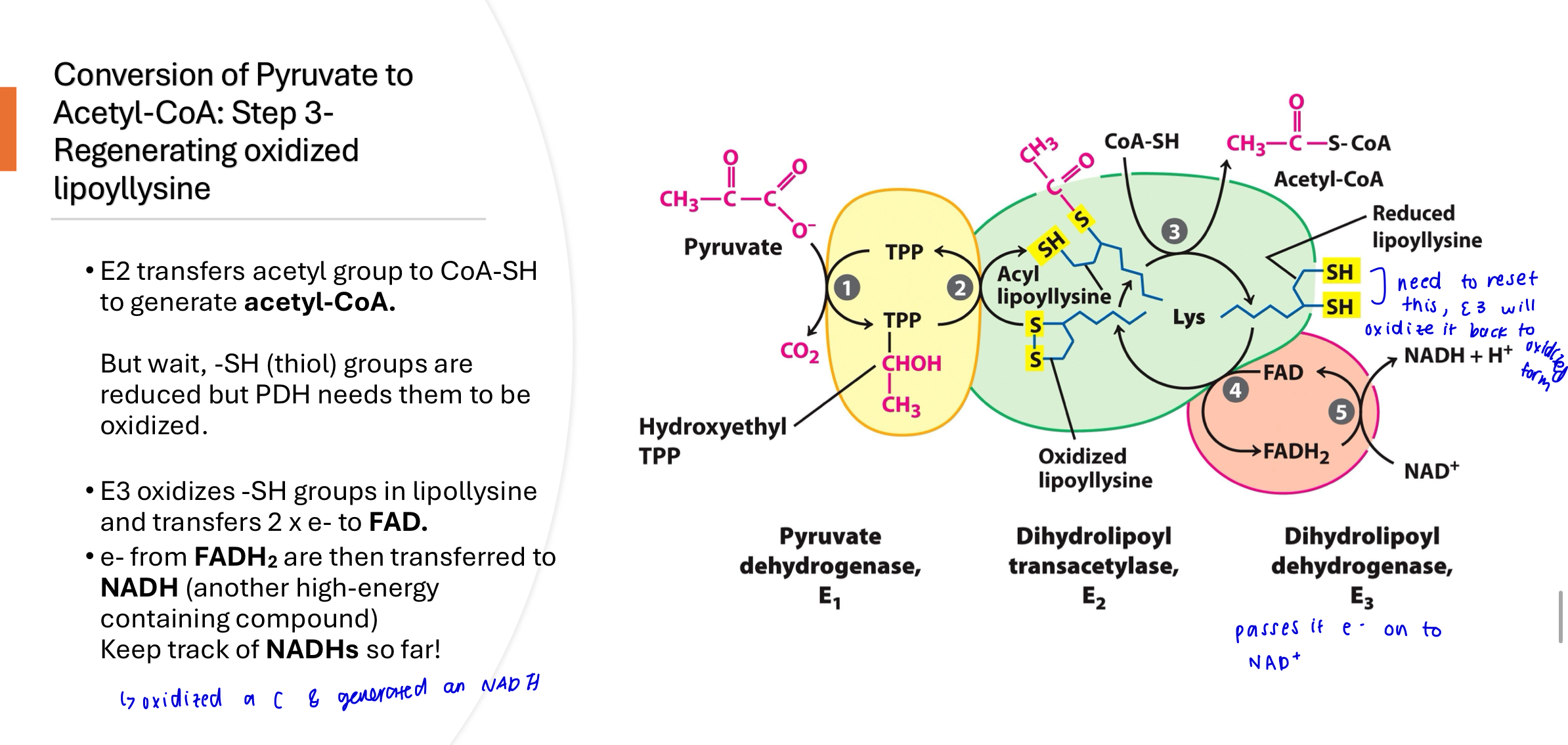
Step 4 of the PDH complex
The reduced lipoyllysine (-SH) is oxidized by E3, transferring electrons to FAD, forming FADH₂. This step regenerates the oxidized form of lipoic acid so it can participate in another catalytic cycle.
step 5 of the PDH complex
FADH₂ transfers its electrons to NAD⁺, producing NADH + H⁺, the second product of the PDH reaction. This allows FAD to be reused and continues the flow of electrons to the electron transport chain.
Overview of the 5 reactions
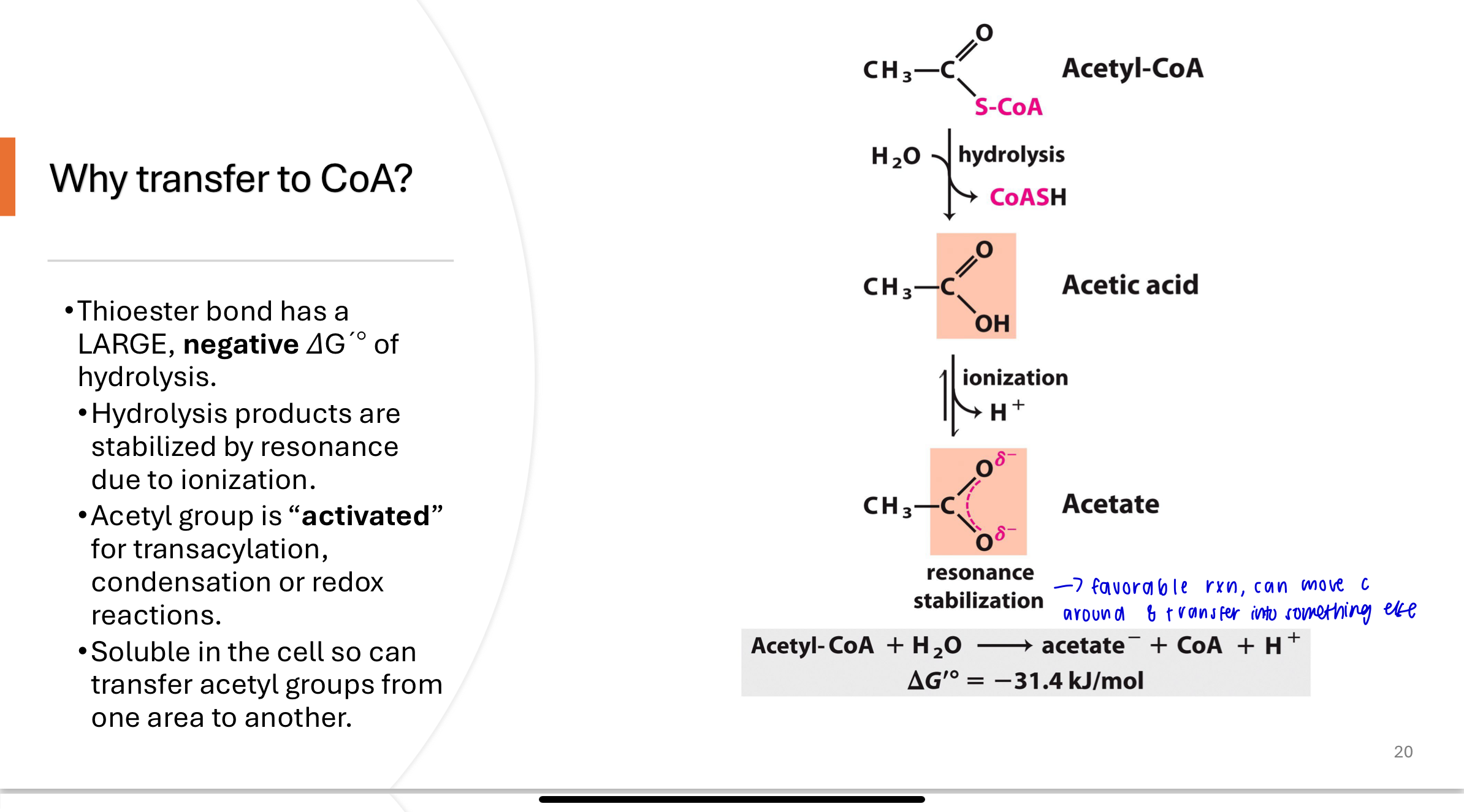
Q: Why is acetyl-CoA considered an “activated” molecule?
The thioester bond in acetyl-CoA has a large negative ΔG′° of hydrolysis, meaning it stores a lot of energy. Hydrolysis products are resonance stabilized, making acetyl-CoA an ideal donor for transacylation, condensation, or redox reactions. Acetyl-CoA is also soluble, enabling it to travel between pathways.
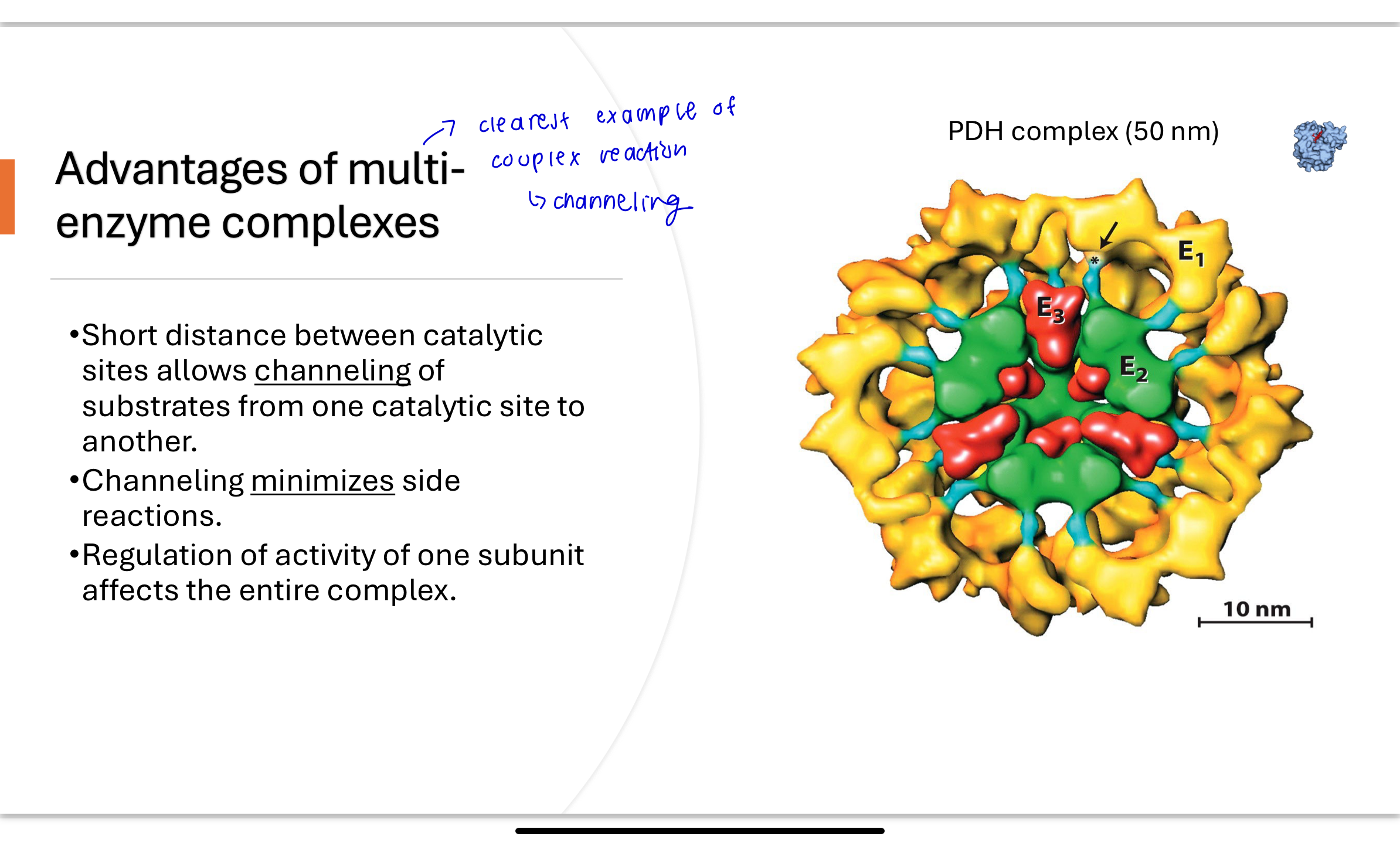
Q: What are the advantages of having PDH as a multi-enzyme complex?
What is the advantage of having three enzymes in ONE complex
Substrate channeling: short distances between active sites prevent diffusion of intermediates
Minimized side reactions
Coordinated regulation: modifying one subunit affects the entire complex
Increases overall reaction speed and efficiency
Where is oxidation occuring?
How is energy of oxidation captured → NADH
Oxidation occurs in Step 2 of the PDH complex reaction. After pyruvate is decarboxylated by E1, the resulting hydroxyethyl group is transferred to lipoic acid on the flexible arm of E2, where it is oxidized to an acetyl group.
The energy from the oxidation of the hydroxyethyl group is captured in two key forms:
As a thioester bond in acetyl-CoA, which has a high-energy hydrolysis potential.
As reducing equivalents in NADH, which are produced after FAD is reduced to FADH₂, and then FADH₂ transfers electrons to NAD⁺, forming NADH.
Both of these products—acetyl-CoA and NADH—carry energy that the cell can later use for ATP production or biosynthetic reactions.
How does the krebs cycle relate to glycolysis
Glycolysis breaks down glucose (6 carbons) into 2 molecules of pyruvate (3 carbons each) in the cytoplasm.
Each pyruvate enters the mitochondria, where the pyruvate dehydrogenase complex (PDH) converts it into acetyl-CoA and releases 1 CO₂.
This acetyl-CoA then enters the Krebs cycle.
So, for every glucose molecule, the cycle turns twice (once per acetyl-CoA).
Q: Why can’t acetyl-CoA carbons be directly oxidized?
The methyl group of acetyl-CoA cannot form a double bond with the carbonyl carbon, making it chemically unreactive for oxidation. Therefore, oxidation must occur indirectly, by attaching acetyl-CoA to oxaloacetate, creating a new structure where hydrogens can be removed from adjacent carbons.
Q: What strategy does the cell use to oxidize the methyl group in acetyl-CoA?
The methyl carbon of acetyl-CoA is combined with oxaloacetate to form a methylene group. This allows stepwise oxidation of carbon atoms, since hydrogens can now be removed from each carbon.
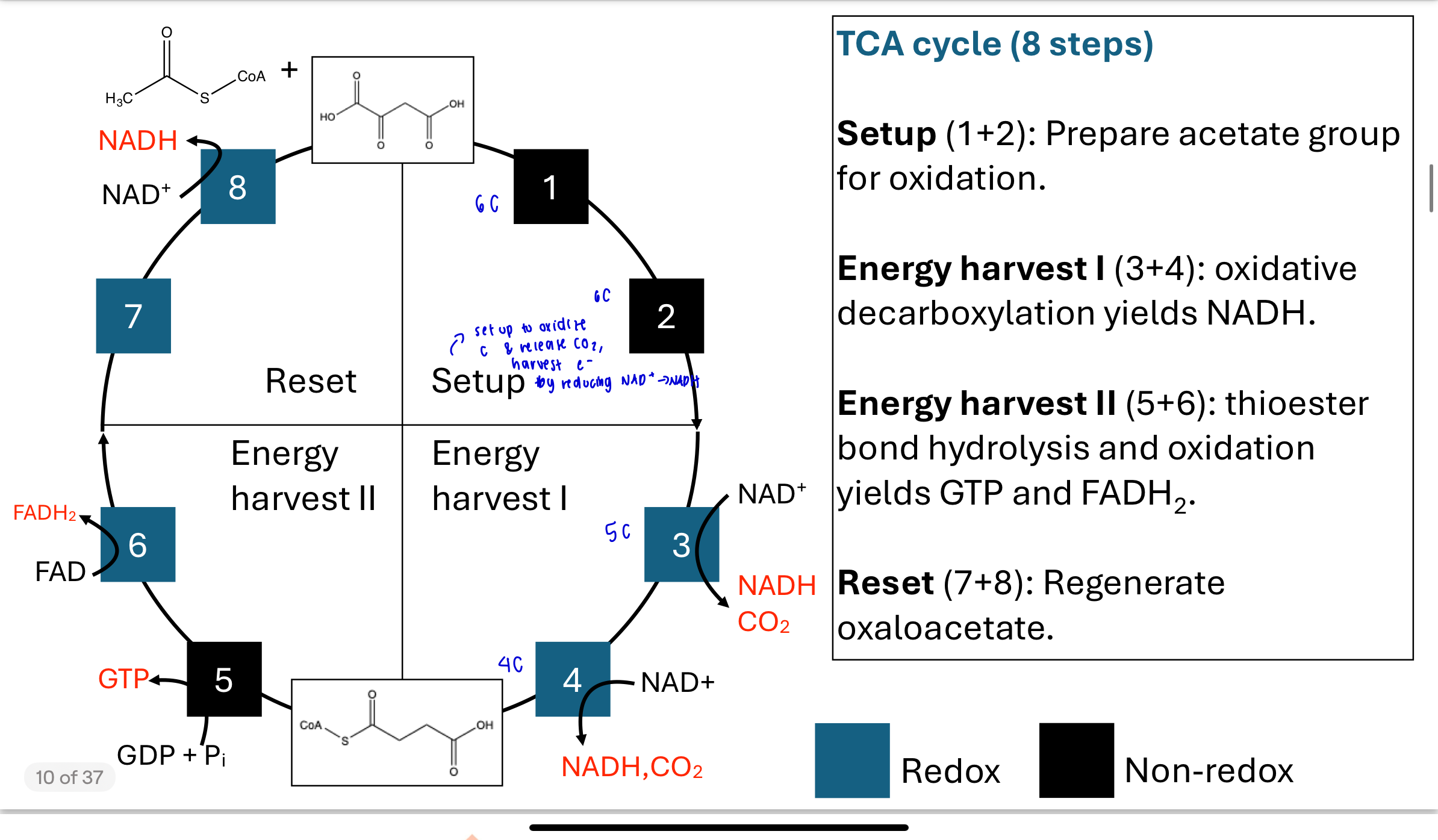
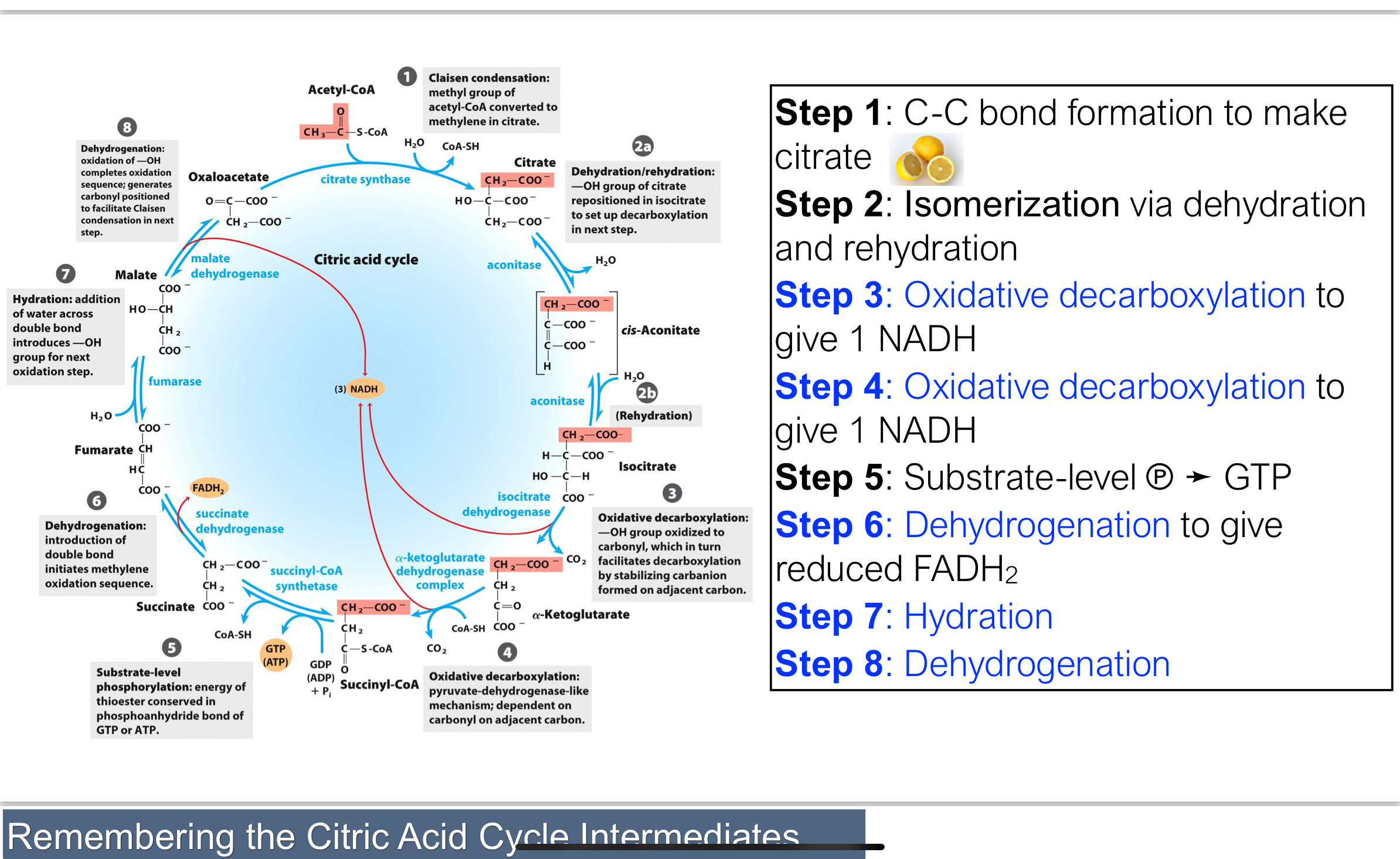
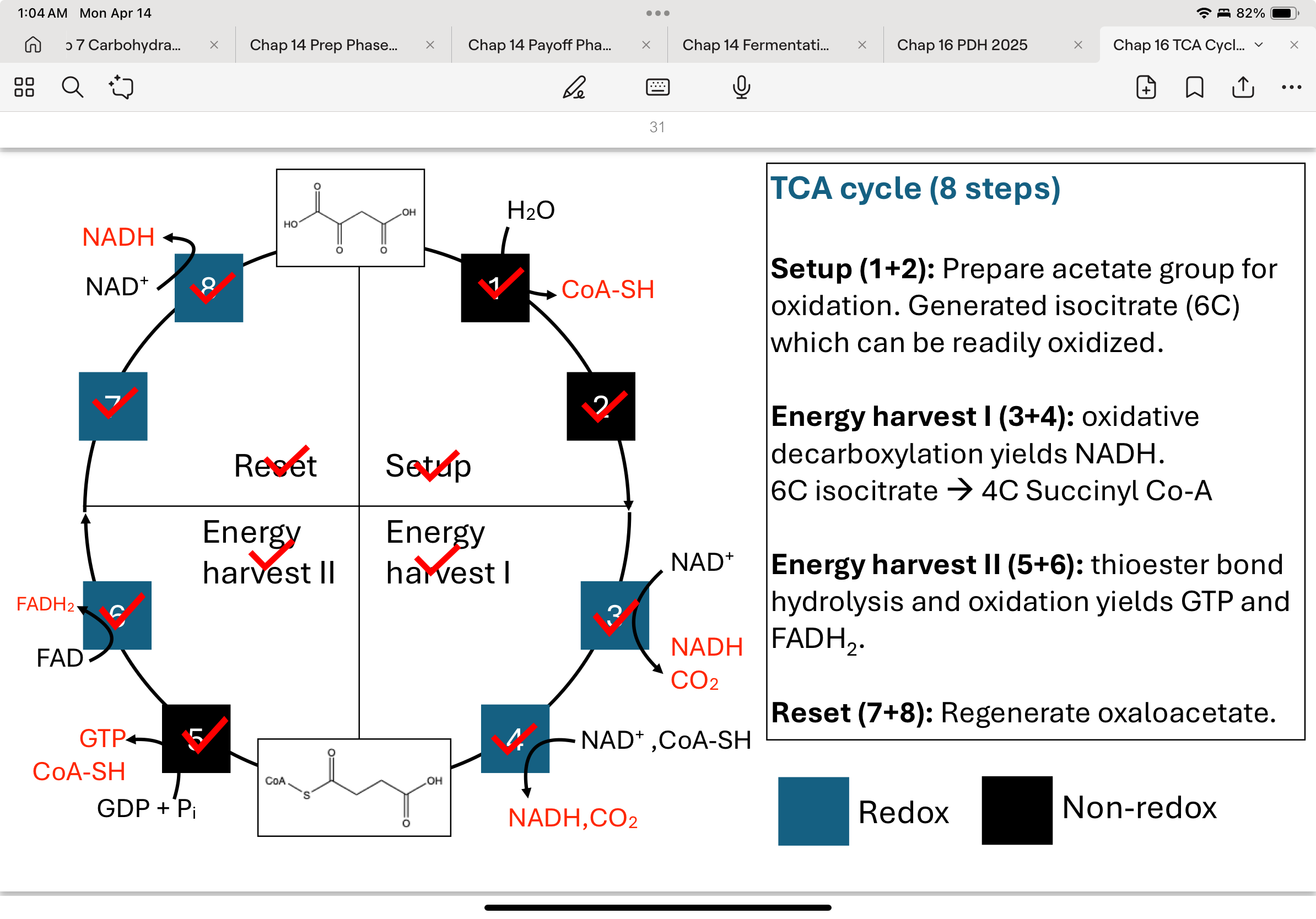
What are the four phases of the 8-step TCA cycle?
Setup (Steps 1–2): Attach acetyl-CoA to oxaloacetate and convert citrate to an oxidizable form.
Energy harvest I (Steps 3–4): Oxidative decarboxylation to release 2 CO₂ and form 2 NADH.
Energy harvest II (Steps 5–6): Cleavage of a thioester bond forms GTP; oxidation forms FADH₂.
Reset (Steps 7–8): Regenerate oxaloacetate to complete the cycle.
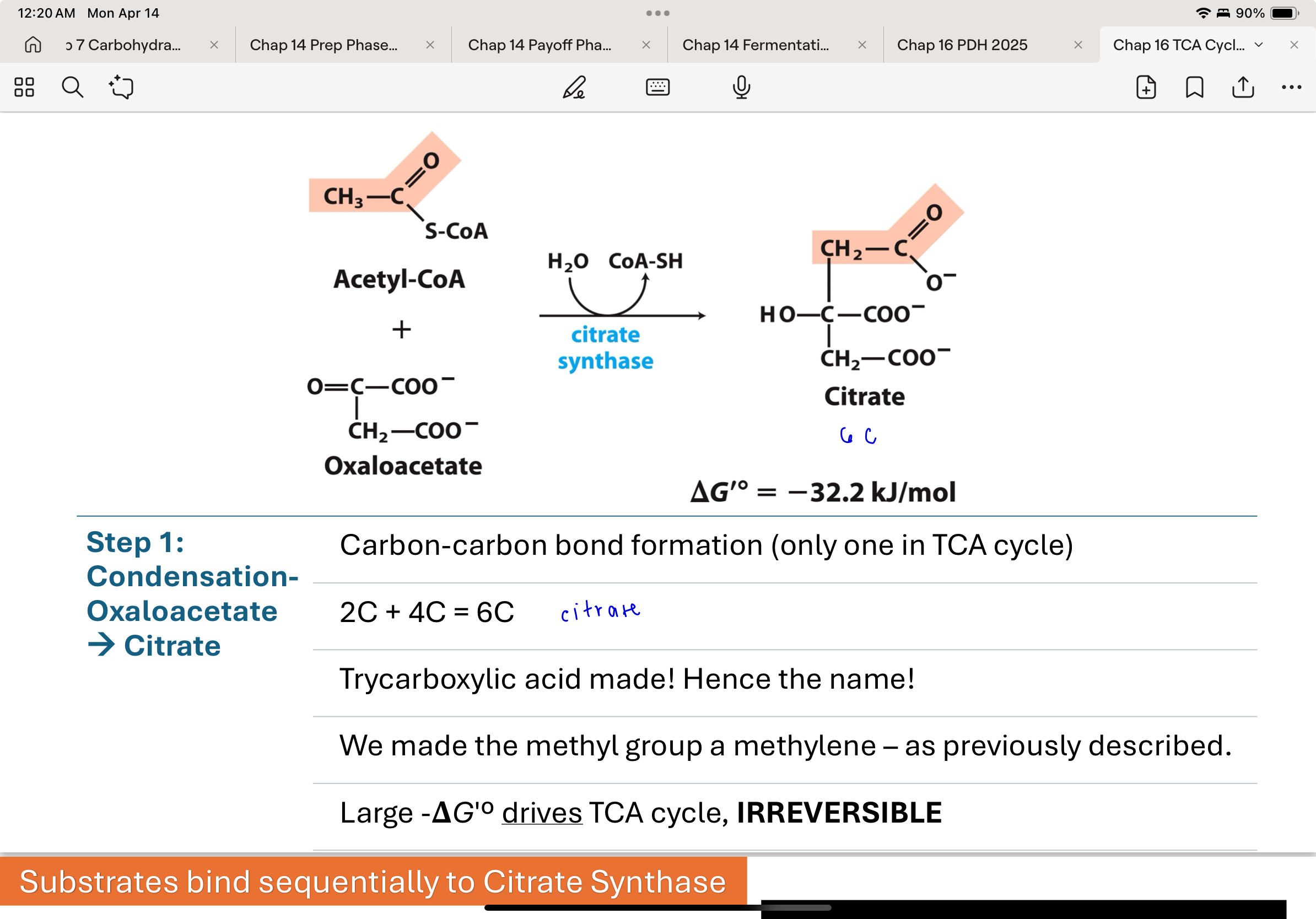
Step 1 of TCA Cycle: Condensation-Oxaloacetate → Citrate
Citrate synthase catalyzes the condensation of acetyl-CoA and oxaloacetate to form citrate.
This is the only carbon-carbon bond-forming step in the cycle.
It's a highly favorable, irreversible reaction that commits the acetyl group to the cycle.
2C+4C = 6C
substrates bind sequentially to citrate synthase
binding of oxaloacetate (OAA) induces conformational change
creates binding site for acetyl-coA
so citrate synthase can bind acetyl coA only after OAA has bound
This prevents early cleavage of the acetyl group without transfer to OAA
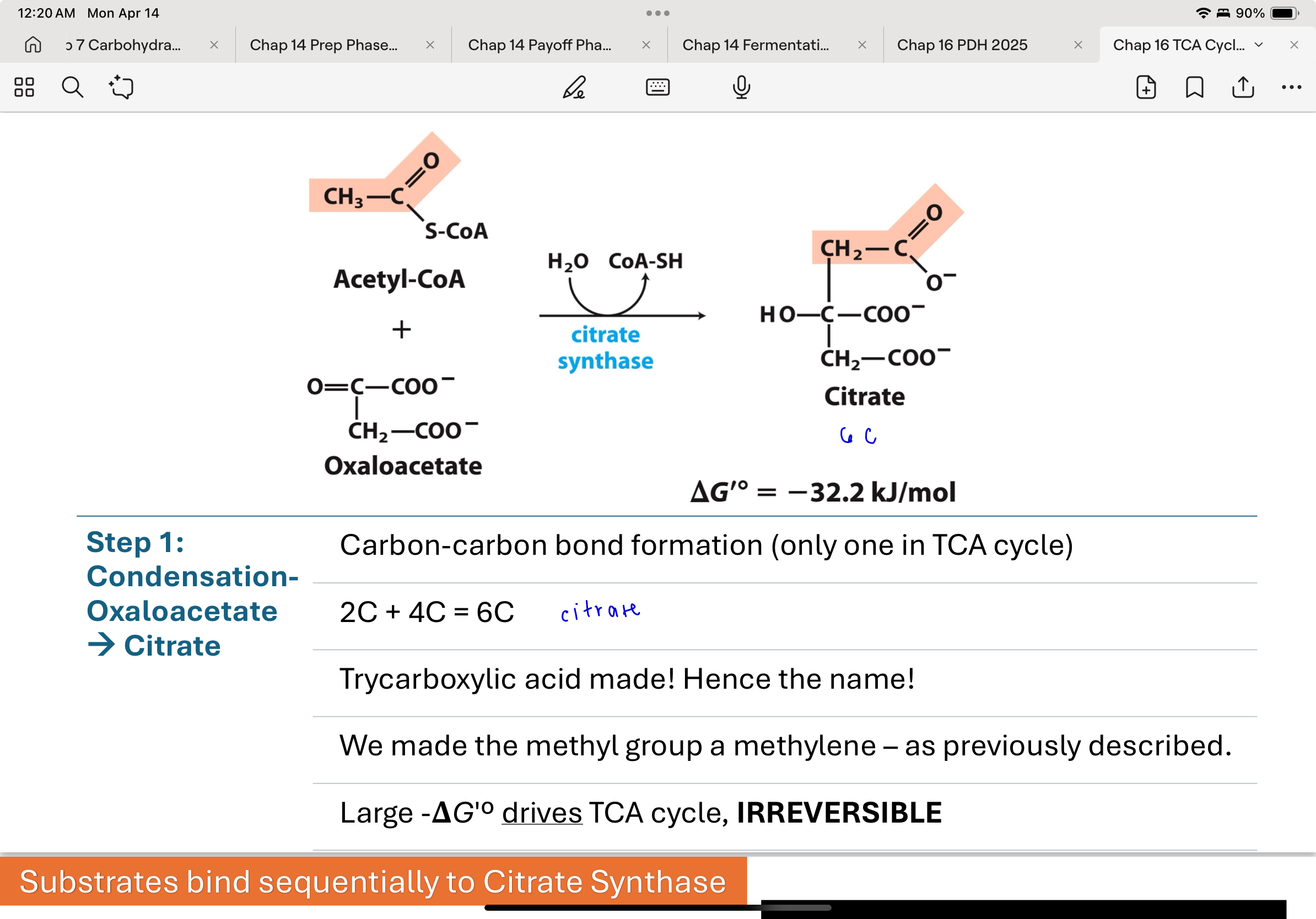
Describe how the substrates of citrate synthase bind to the enzyme. Why does the order of substrate binding matter for this enzyme? What could happen if this was not controlled? (4pts)
- Oxaloacetate (OAA) binds first and then Acetyl-CoA binds to the active site in that order.
- Since cleaving the CoA from the acetyl group is favorable this prevents premature cleavage and release of the acetyl group before it can be joined to OAA.
- If that happened those carbons would be lost to the TCA cycle and energy would not be harvested through oxidation
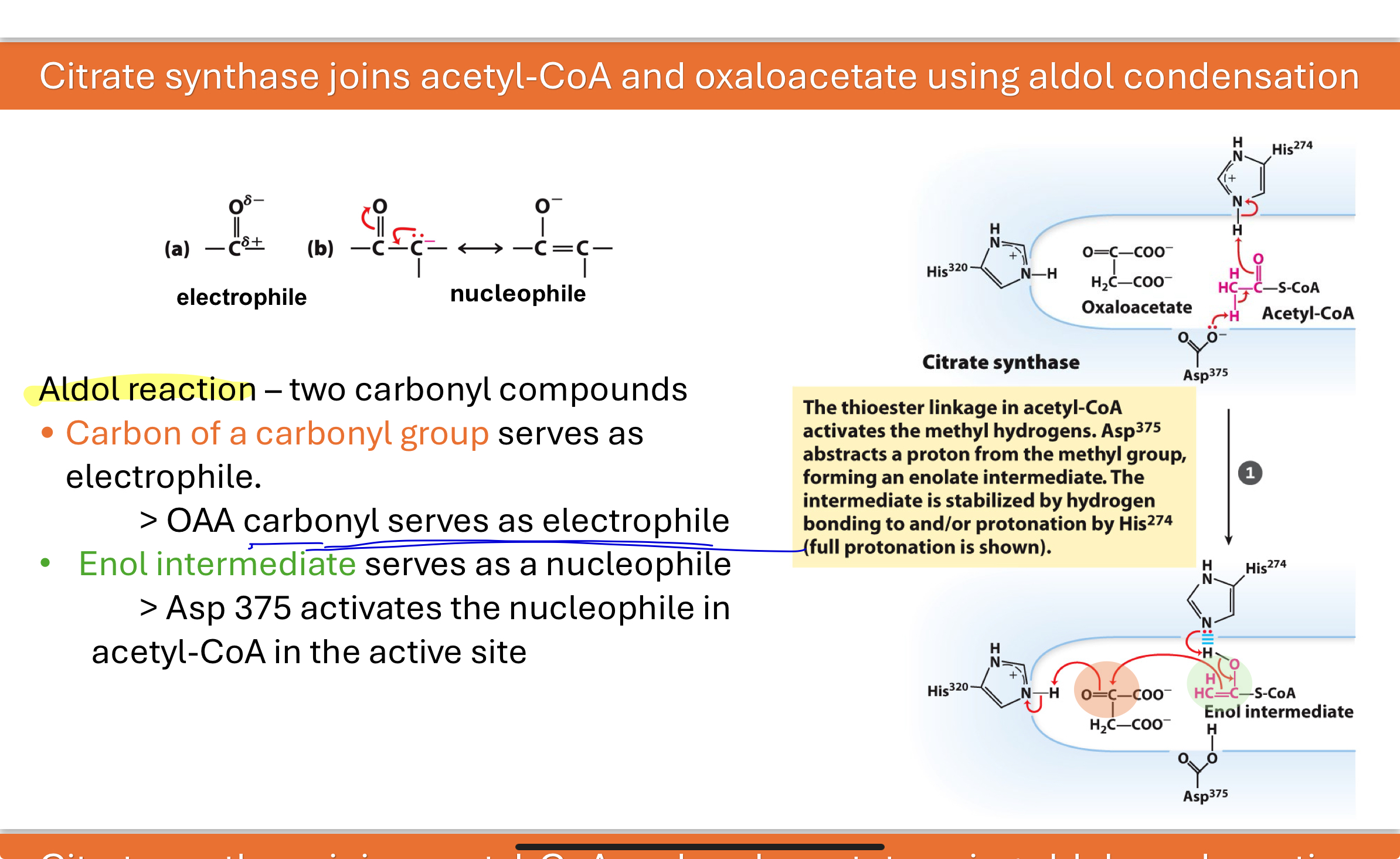
What kind of reaction occurs during the formation of citrate?
An aldol condensation occurs.
The carbonyl carbon of oxaloacetate acts as an electrophile, and an enol intermediate formed from acetyl-CoA acts as the nucleophile.
Enzyme residues Asp375 and His320 assist in this bond formation and stabilization.
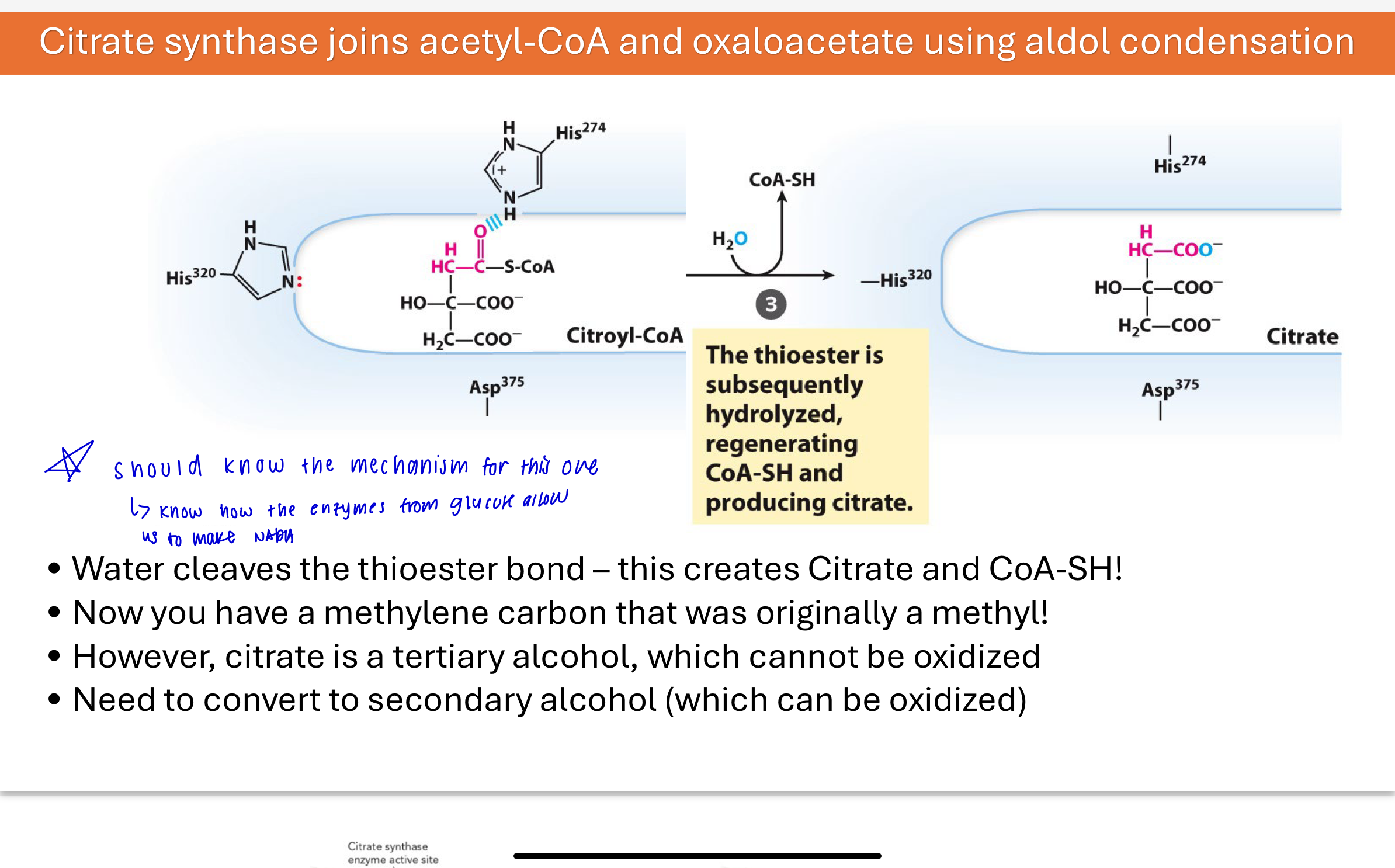
Q: What happens after citroyl-CoA is formed in Step 1?
Water hydrolyzes the thioester bond in citroyl-CoA, forming citrate and releasing CoA-SH. This transforms the original methyl carbon from acetyl-CoA into a methylene, which can now be oxidized.
Q: What happens in Step 2 of the TCA cycle?
Citrate is isomerized to isocitrate by first eliminating water (dehydration) to form a cis-aconitate, then adding water (hydration) to form isocitrate, a secondary alcohol.
This conversion is reversible and thermodynamically unfavorable, but is pulled forward by product removal.
convert it from tertiary to secondary done by aconitase
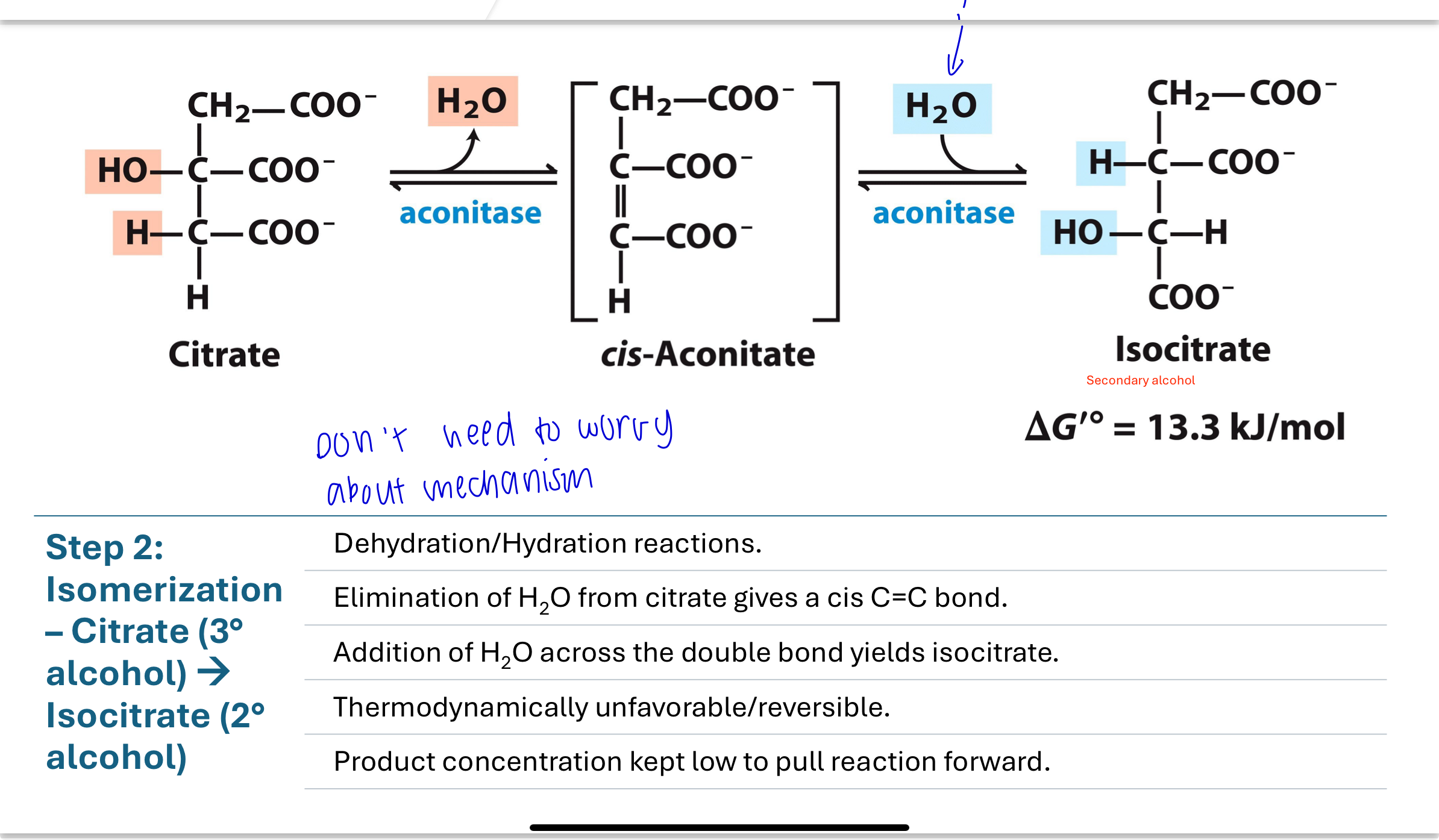
Q: What is the purpose of isomerizing citrate to isocitrate?
Citrate is a tertiary alcohol, which cannot be oxidized. By converting it to isocitrate, a secondary alcohol, the molecule becomes ready for the first oxidation step of the cycle.
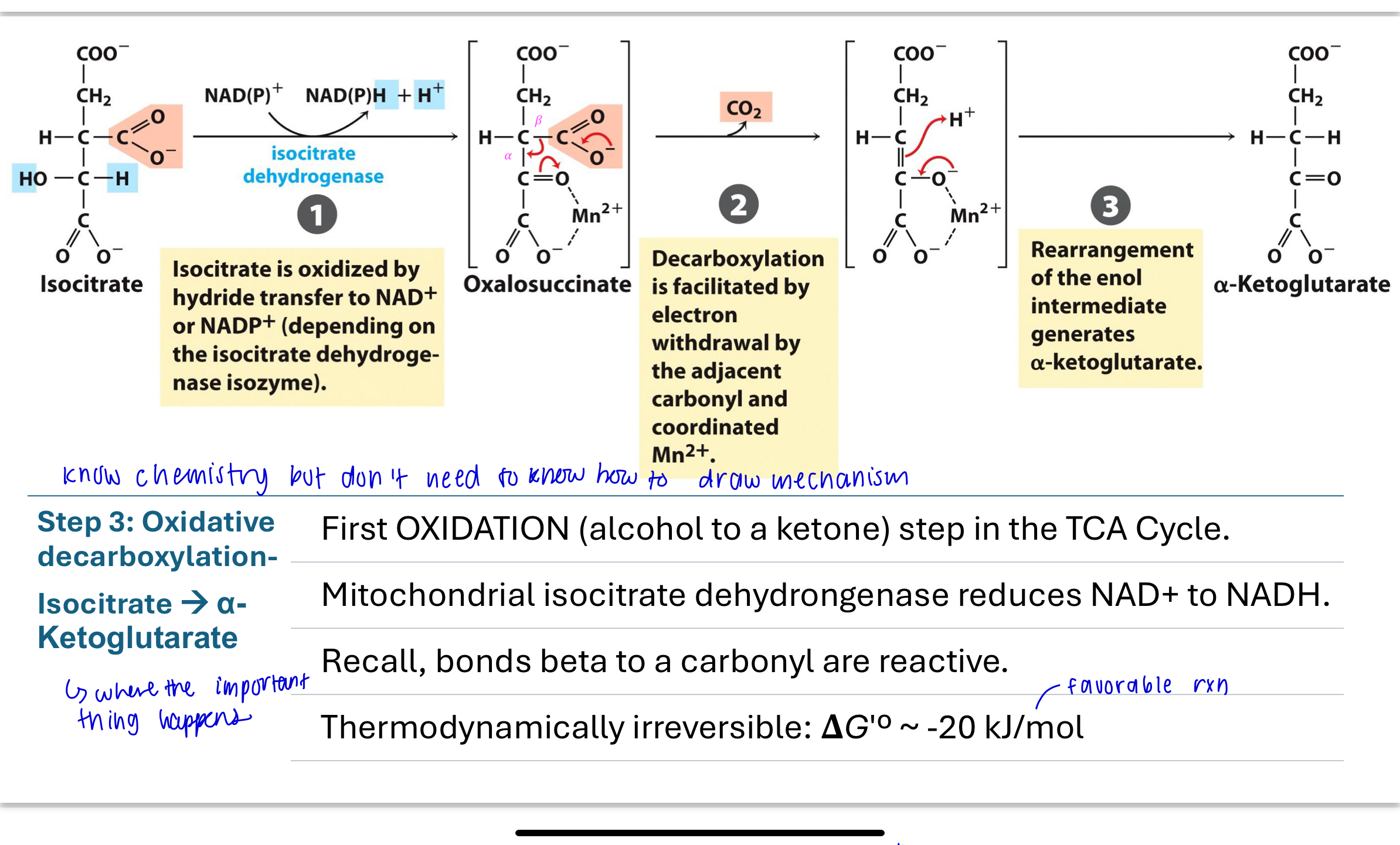
What happens in Step 3 of the TCA cycle?
Isocitrate undergoes oxidative decarboxylation to form α-ketoglutarate.
The alcohol is oxidized to a ketone, and CO₂ is released. Isocitrate dehydrogenase reduces NAD⁺ to NADH.
This is the first redox reaction and is irreversible due to a large negative ΔG′°.
know the chemistry, don’t need to know mechanism
What happens in Step 4 of the TCA cycle?
α-Ketoglutarate is oxidized to succinyl-CoA via a multi-enzyme complex similar to pyruvate dehydrogenase. This reaction releases CO₂ and generates another NADH. It’s another irreversible oxidative decarboxylation, reducing the carbon count from 5 to 4.
The goal of respiration is to oxidize the carbons from glucose in a stepwise fashion to slowly harvest the energy. By which point (reaction number/step) in which process does this occur? (
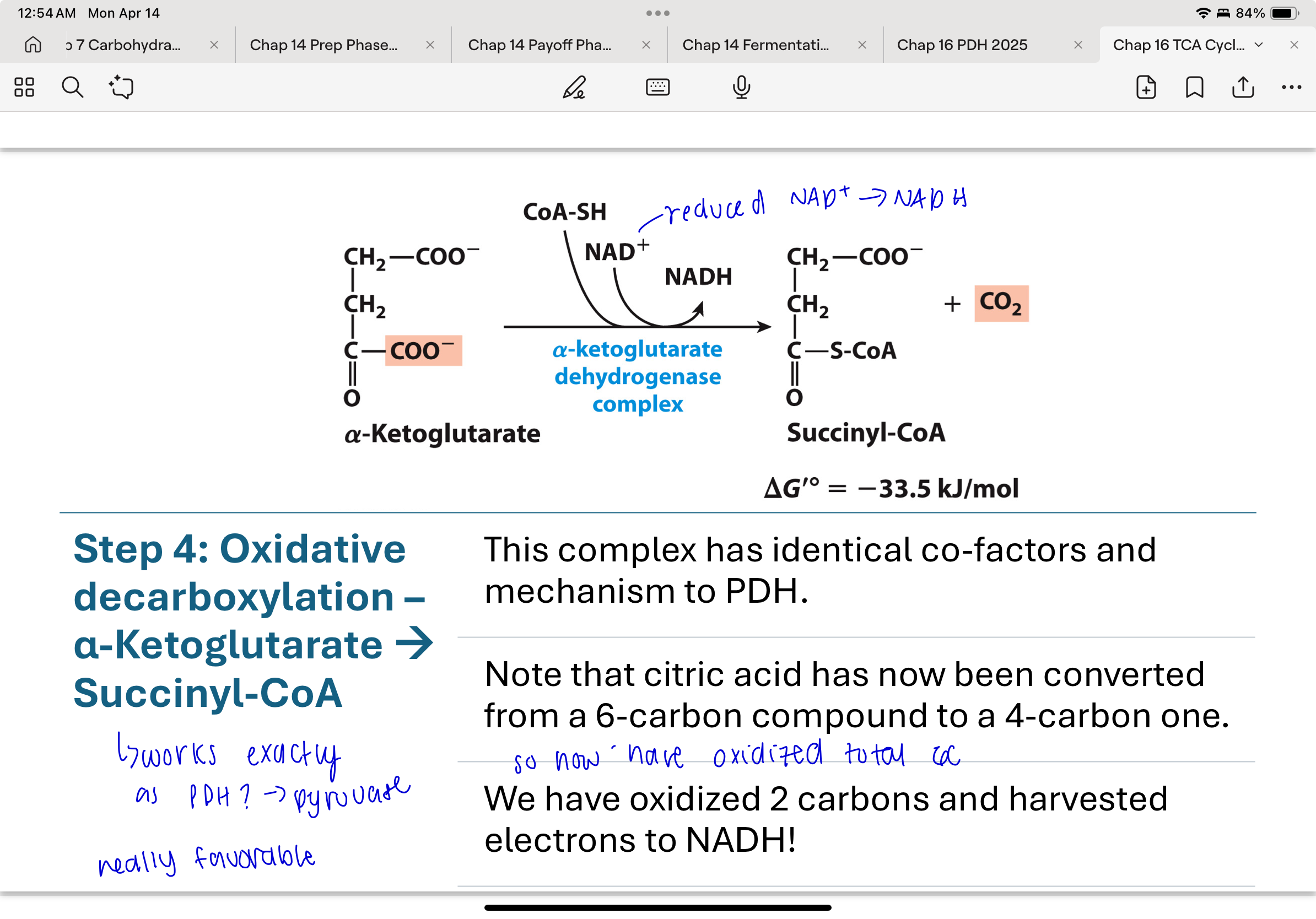
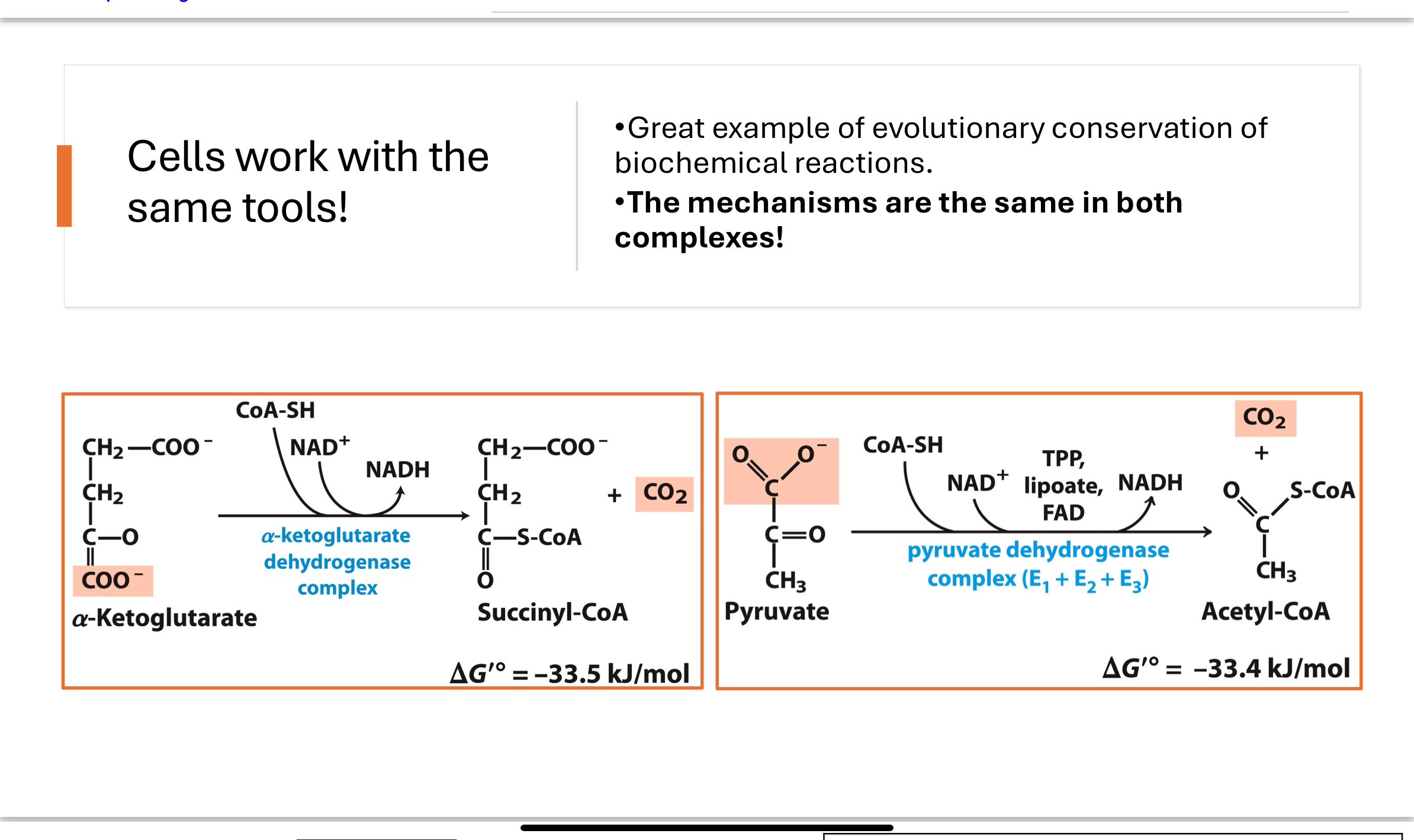
What enzyme complex catalyzes Step 4, and what is its evolutionary significance?
The α-ketoglutarate dehydrogenase complex shares the same cofactors (TPP, lipoic acid, FAD, NAD⁺, CoA-SH) and mechanism as pyruvate dehydrogenase. This is an example of evolutionary conservation of enzyme function.
What happens in Step 5 of the TCA cycle?
The thioester bond in succinyl-CoA is hydrolyzed, releasing CoA-SH (succinyl-CoA synthetase) and transferring a phosphate to GDP, forming GTP (a form of ATP). This is called substrate level phosphorylation (similar in glycolysis) → makes succinate
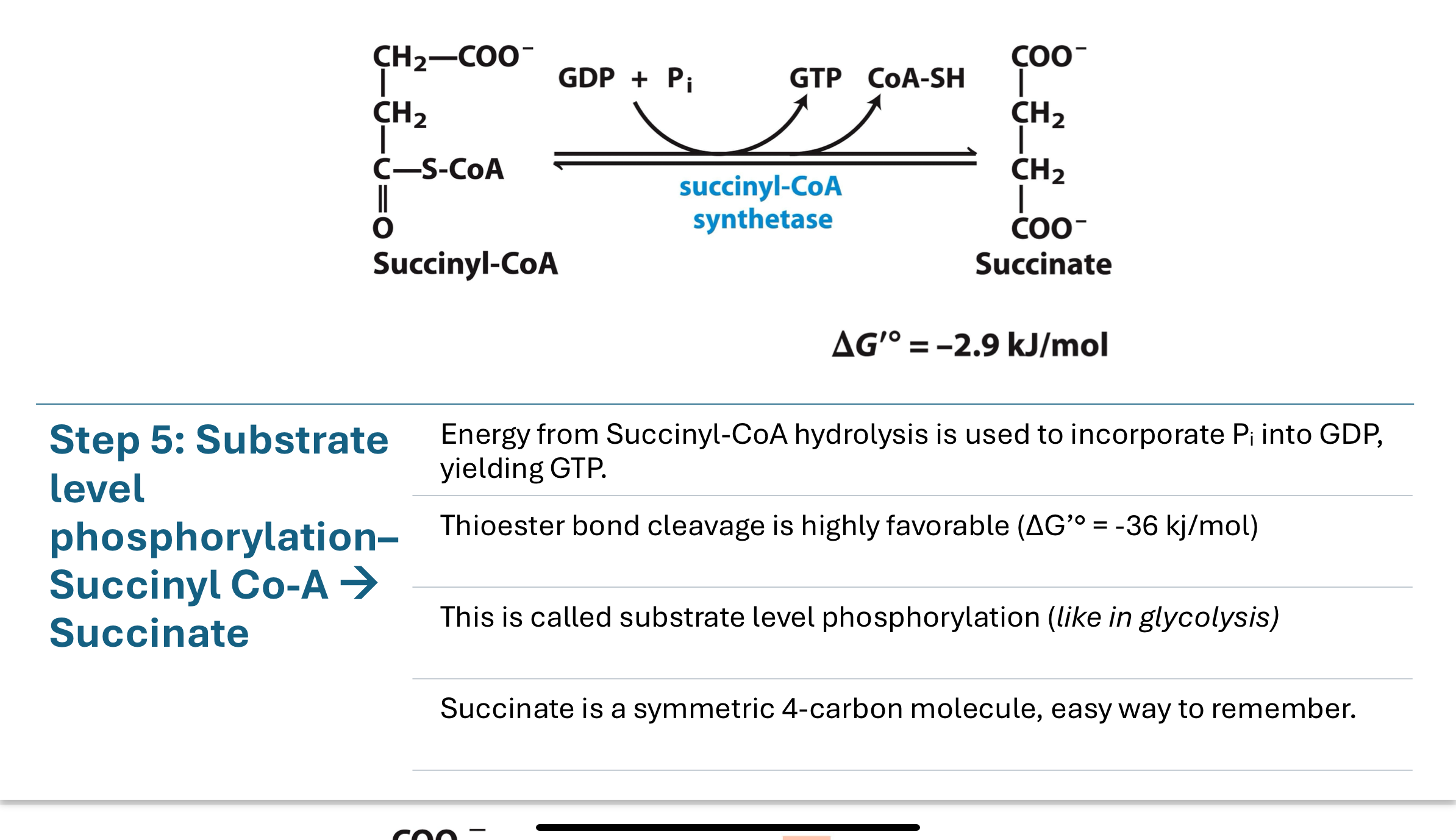
step 6 in the TCA cycle
turns succinate into fumarate by using succinate dehydrogenase
reduction of FAD to FADH2
at/near equilibrium — reversible
fumarate concentration is kept low to pull reaction forward
DRAW out the reaction of the TCA cycle that that shares an enzyme with the ETC. Indicate clearly what pathway each product continues in. Molecular structures (except for electron carriers) are expected in this answer except
Fumarate will continue through the TCA cycle and FADH2 will pass it’s electrons through the ETC (they go to Q if their answer gets that specific).
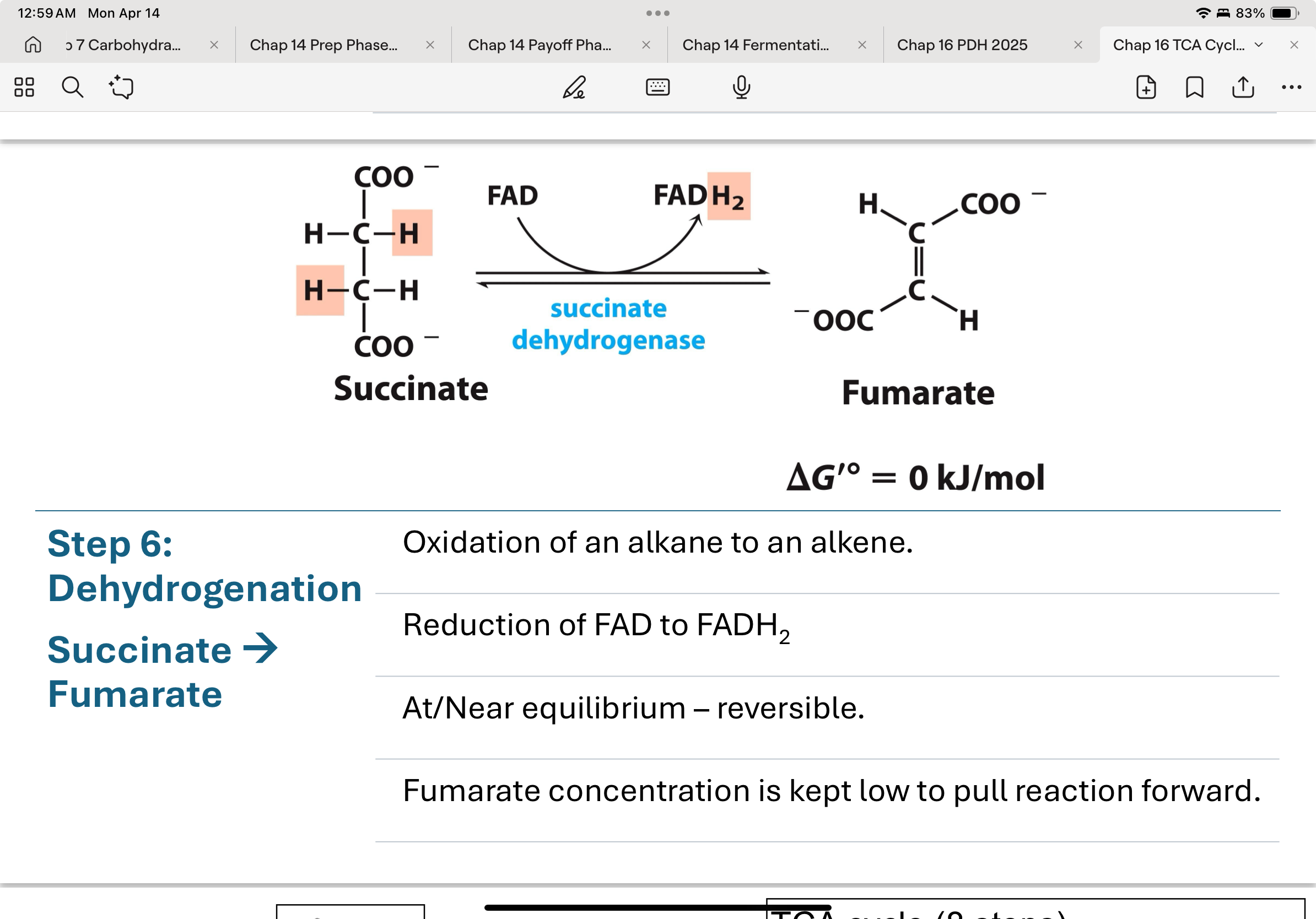
step 7 in the TCA cycle
fumarate is turned into L-malate by fumarase
goal or reactions 7 and 8 are to regenerate OAA to keep the cycle going
addition of water across the double bond
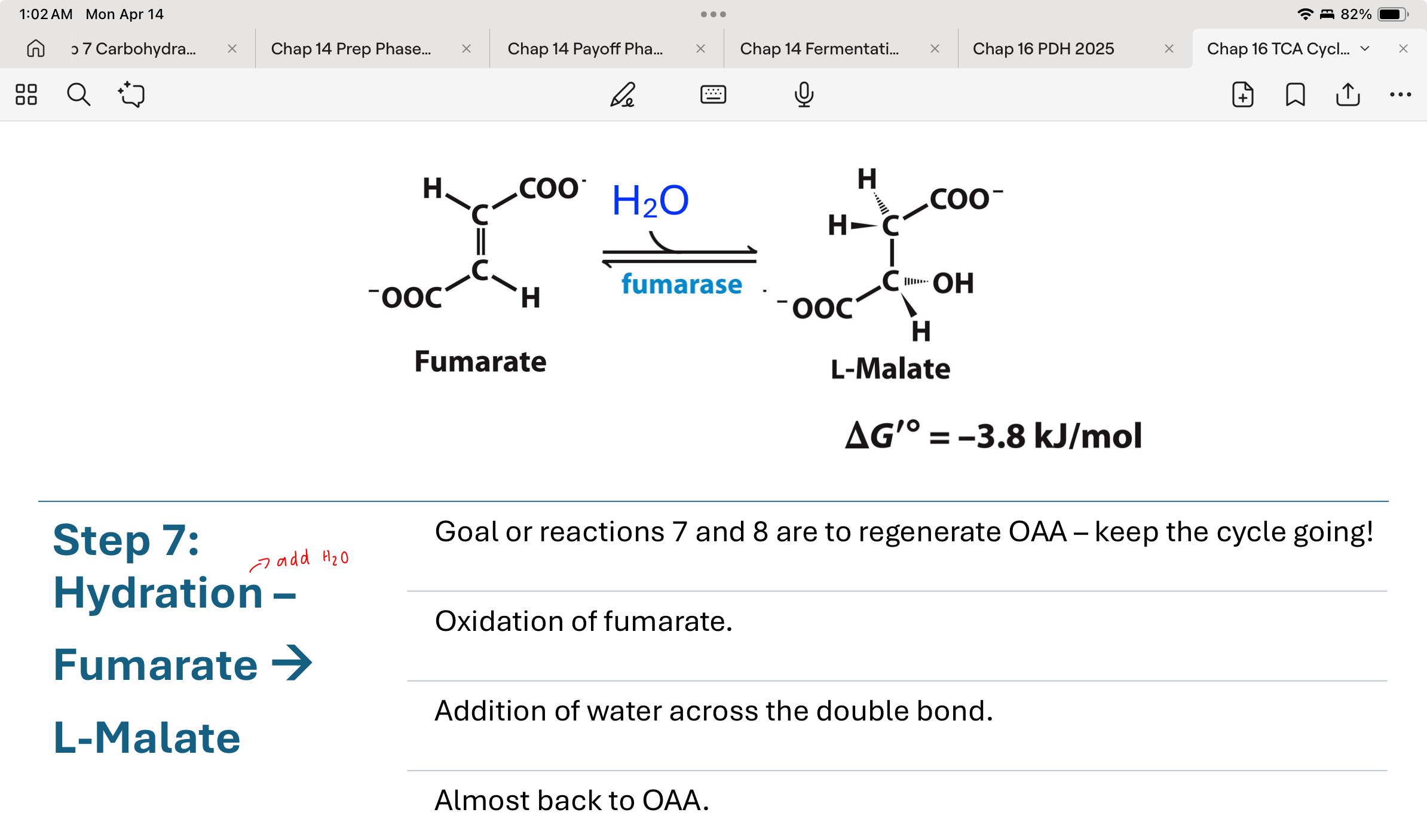
step 8 in the TCA cycle
L-malate turned into Oxaloacetate by malate dehydrogenase
reduction of NAD+ to NADH
Regeneration of OAA
thermodynamically unfavorable so [oxaloacetate] is kept very low by immediate use in Step 1 to pull the reaction forward.
![<p>L-malate turned into Oxaloacetate by malate dehydrogenase </p><ul><li><p>reduction of NAD+ to NADH</p></li><li><p>Regeneration of OAA </p></li><li><p>thermodynamically unfavorable so <strong>[oxaloacetate] is kept very low</strong> by immediate use in Step 1 to pull the reaction forward.</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/bc08704b-5e2d-4927-b524-66fab22352cd.png)
TCA cycle over view
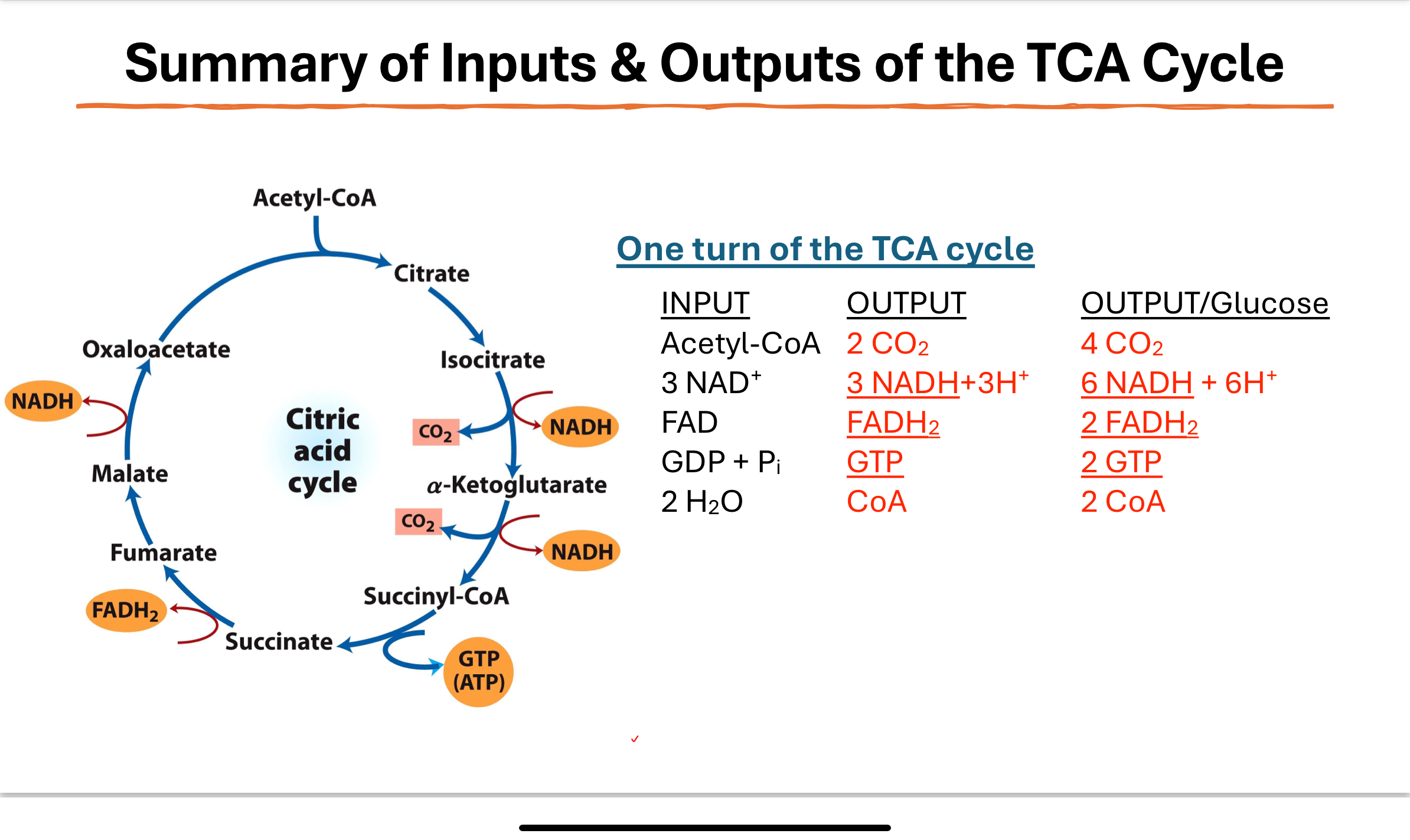
What are the inputs and outputs of one TCA cycle turn?
Inputs: Acetyl-CoA, 3 NAD⁺, FAD, GDP + Pi, 2 H₂O
Outputs: 2 CO₂, 3 NADH + H⁺, FADH₂, GTP, CoA-SH
Per glucose: Double all values (because 1 glucose = 2 pyruvate = 2 acetyl-CoA)
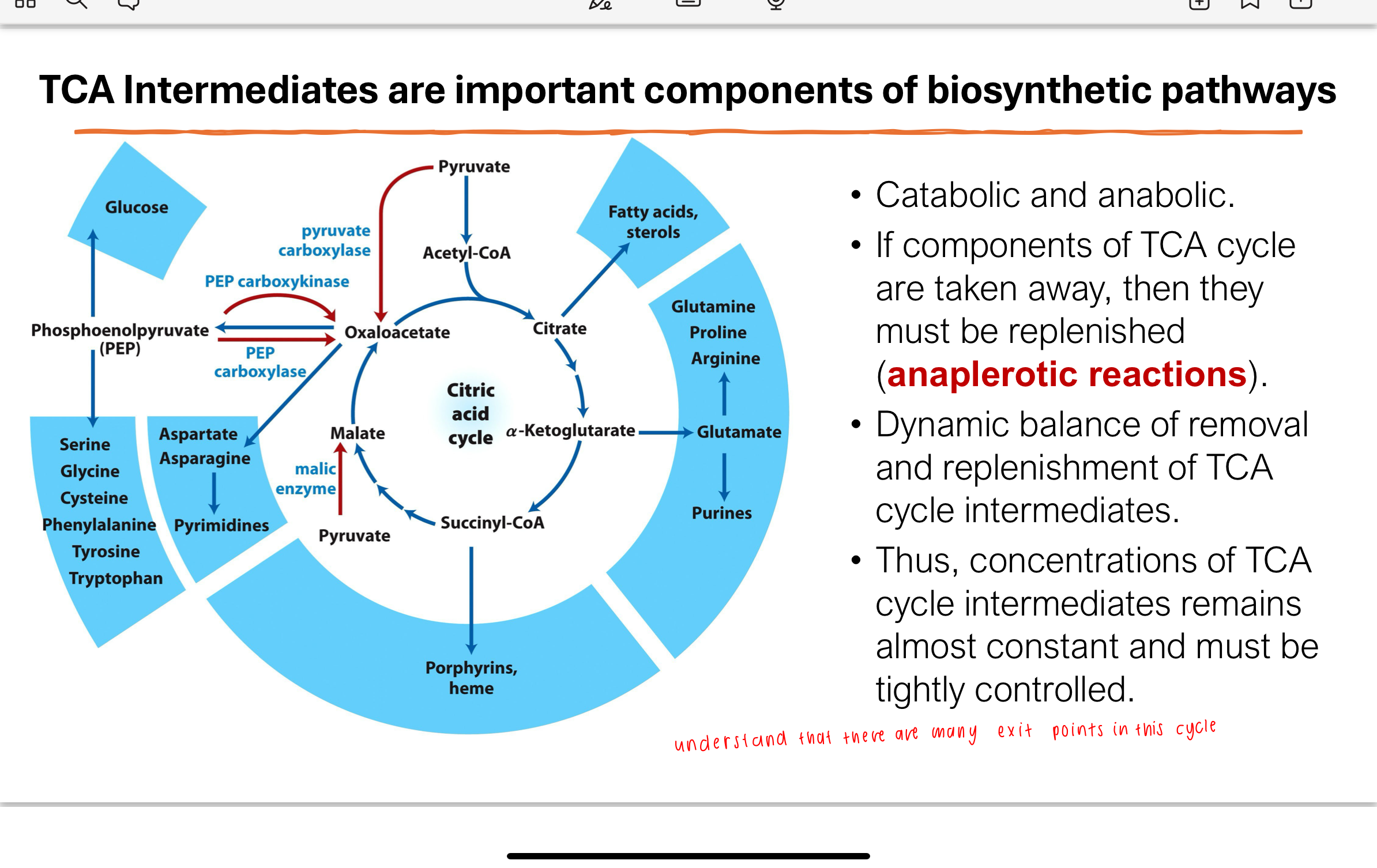
Q: How do TCA cycle intermediates function in biosynthesis?
TCA intermediates are used in biosynthetic pathways (amino acid, nucleotide, heme synthesis, etc.). Their removal must be balanced by anaplerotic reactions that replenish them. This maintains a dynamic equilibrium where the concentrations of intermediates stay fairly constant.