Dibartola Chapter 1: Applied Physiology of Body Fluids in Dogs and Cats
1/161
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
162 Terms
What percent of an adult animal’s body weight is water in health?
60%
What percent of a neonate’s body weight is total body water?
80%
Calculating Fluid Needs Based on Weight vs Lean Body Mass
Fat has a lower water content than lean tissue so fluid needs should be estimated on the basis of lean body mass to avoid overhydration
Body weight is a reasonable estimate of lean body mass in thin patients
What % of body weight is due to fat in normal small animal patients?
20%
What % of body weight is fat in morbidly obese patients?
~30%
Lean Body Mass Equations
Normal body weight x 0.8 = lean body mass
Obese body weight x 0.7 = lean body mass
Thin body weight x 1.0 - lean body mass
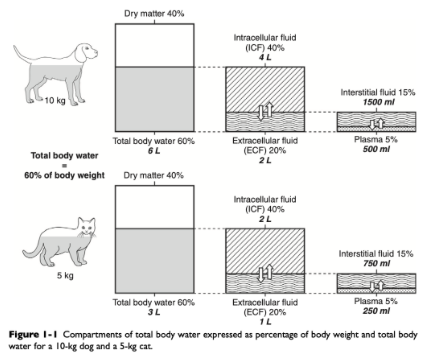
What is the largest volume of fluid in the body?
Intracellular fluid
What percent of body weight is intracellular fluid?
~40%
What fraction of total body water is intracellular fluid?
~2/3
Composition of ICF vs ECF
What fraction of total body water is extracellular fluid?
~1/3
Where does loss of fluids occur initially from in most disease states?
ECF
What % of lean body weight is ECF?
Estimates vary widely (15-30%)
Most accurate estimate is ~27% of lean body weight
60:40:20 rule
60% of body weight is water
40$ of body weight is ICF
20% of body weight is ECF
Where is the interstitial fluid?
Spaces surrounding cells
What % of body weight and total body water is interstitial fluid?
~15% of body weight
~24% of total body water
Subcompartments of the Extracellular Fluid (ECF)
Interstitial fluid
Intravascular component (plasma)
Transcellular fluid
Where is the intravascular fluid located?
Within blood vessels
Most of the intravascular fluid is plasma
What % of body weight, body water, and lean body mass is intravascular fluid?
~5% of body weight
~8-10% total body weight
Blood volume is a function of lean body mass, ~8% body weight in a horse
What is transcellular fluid?
Fluids produced by specialized cells to form cerebrospinal fluid, gastrointestinal fluid, bile, glandular secretions, respiratory secretions, and synovial fluid
What % of body weight and total body water is transcellular fluid?
~1% of body weight
~2% total body water
Simplified Distribution of Total Body Water for Fluid Therapy
ICF ~2/3 of total body water
ECF ~1/3 of total body water
ISF ~3/4 of ECF
Intravascular fluid is ~1/4 of ECF
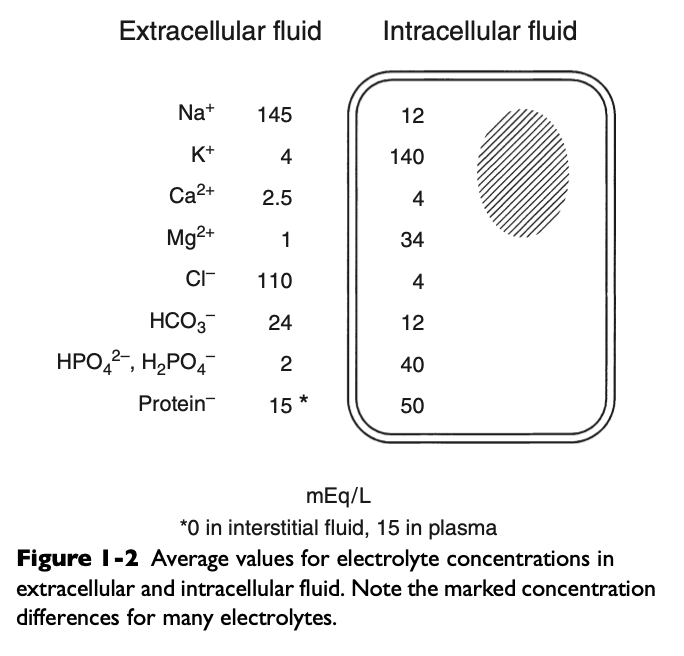
Distribution of Body Solutes
Healthy vascular endothelium is relatively impermeable to the cellular components of blood and plasma proteins so the volume of distribution of cells and proteins is the plasma space
The vascular endothelium is freely permeable to ionic solutes and the concentration of these ions is almost the same in ISF as in plasma
Cell membranes maintain intracellular solutes at very different concentrations from those of the ECF
Plasma Na+
142 mEq/L
Plasma K+
4.3 mEq/L
Plasma Ca2+
2.5 mEq/L
Plasma Mg2+
1.1 mEq/L
Plasma Cl-
104 mEq/L
Plasma HCO3-
24 mEq/L
Plasma HPO42-, H2PO4-
2 mEq/L
Plasma Proteins
14 mEq/L
Plasma Other Anions
5.9 mEq/L
Plasma Water Na+
152.7 mEq/L
Plasma Water K+
4.6 mEq/L
Plasma Water Ca2+
2.7 mEq/L
Plasma Water Mg2+
1.2 mEq/L
Plasma Water Cl-
111.9 mEq/L
Plasma Water HCO3-
25.8 mEq/L
Plasma Water HPO42-, H2PO4-
2.2 mEq/L
Plasma Water Proteins
15 mEq/L
Plasma Water Other Anions
6.3 mEq/L
Interstitial Fluid Na+
145.1 mEq/L
Interstitial Fluid K+
4.4 mEq/L
Interstitial Fluid Ca2+
2.4 mEq/L
Interstitial Fluid Mg2+
1.1 mEq/L
Interstitial Fluid Cl-
117.4 mEq/L
Interstitial Fluid HCO3-
27.1 mEq/L
Interstitial Fluid HPO42-, H2PO4-
2.3 mEq/L
Interstitial Fluid Proteins
0 mEq/L
Interstitial Fluid Other Anions
6.2 mEq/L
Intracellular Fluid - Skeletal Muscle Cell Na+
12 mEq/L
Intracellular Fluid - Skeletal Muscle Cell K+
140 mEq/L
Intracellular Fluid - Skeletal Muscle Cell Ca2+
4.0 mEq/L
Intracellular Fluid - Skeletal Muscle Cell Mg2+
34 mEq/L
Intracellular Fluid - Skeletal Muscle Cell Cl-
4 mEq/L
Intracellular Fluid - Skeletal Muscle Cell HCO3-
12 mEq/L
Intracellular Fluid - Skeletal Muscle Cell HPO42-, H2PO4-
40 mEq/L
Intracellular Fluid - Skeletal Muscle Cell Proteins
50 mEq/L
Intracellular Fluid - Skeletal Muscle Cell Other Anions
84 mEq/L
Why is there a slightly increased concentration of cations and anions in interstitial fluid compared with plasma water?
Primarily because of the presence of negatively charged proteins in plasma
Gibbs-Donnan Equilibrium
Determines the equilibrium concentrations of permeable anions and cations across the vascular endothelium
Occurs because negatively charged, nondiffusible proteins affect the distribution of other small charged solutes
In clinical practice, the difference in concentrations of anions and cations across the vascular endothelium is negligible and the effects of the Gibbs-Donnan equilibrium are usually ignored
In clinical practice, plasma concentrations of solutes are considered to reflect solute concentrations throughout the ECF
What is the most abundant cation in the ECF?
Na+
Where is most of the body Na+ located?
The extracellular space
How much of body Na+ is exchangable?
~70% of body Na+ in humans is exchangeable and 30% is fixed as insoluble salts in bone
Only exchangeable solutes are osmotically active
Movement of Na+ In and Out of Cells
Cell membranes are permeable to Na+ which tends to diffuse into cells
In health, cell membrane Na+K+ ATPase actively removes Na+ from cells, maintaining a steep extracellular to intracellular concentration gradient for Na+
What are the most abundant anions in the ECF?
Cl- and HCO3-
Where is Cl- mostly located?
Volume of distribution of Cl- is primarily the ECF volume
Where is HCO3- located?
HCO3- is present in all body fluids and can be generated from CO2 and H2O in the presence of carbonic anhydrase
What are the primary cations in the ICF?
K+ and Mg2+
Where is most of the body K+ located?
In the ICF where K+ is the most abundant cation
Movement of K+ In and Out of Cells
Cell membranes are permeable to K+
Cell membrane Na+K+ ATPase maintains the concentration gradient between ICF and ECF by moving K+ into cells against a concentration gradient
The ratio of intracellular to extracellular K+ concentration is important in generating and maintaining the cell membrane potential at approximately -70 mV
How much of K+ is exchangeable?
Almost 100% of body K+ in humans is exchangeable
What are the predominant anions in the ICF?
Organic phosphates and proteins
Atomic Mass (Relative Atomic Mass or Atomic Weight)
Most naturally occurring elements consist of one or more isotopes of that element, each of which has a different mass
Atomic mass - an average mass based on the distribution of stable isotopes for an element, determined by the weight of that element relative to the weight of the 12C isotope of carbon, which is defined as 12.000
Usually reported with no units are as atomic mass units
Molecular Mass (Molecular Weight)
The sum of the atomic masses of the atoms that form the compound
Formula Weight
Ionic compounds do not really form molecules so a more appropriate term for the mass of these substances is formula weight
Mole
6.023 x 1023 particles
Defined as the number of atoms in exactly 12 g of 12C
One mole of a substance weighs its molecular weight in grams
Molar Mass
The mass in grams of 1 mole of a substance
Numerically equivalent to atomic or molecular weight but are reported in grams
Molality and Molarity
Molality - the number of moles of solute per kilogram of solvent
Molarity - the number of moles of solute per liter of solution
Molarity and molality of most biologic solutions are approximately equal because the density of water is 1 kg/L
Slight difference between molarity and molality of a substance in plasma is because of nonaqueous proteins and lipids
In body fluids this difference is relatively unimportant and the terms are often used interchangeably
Concentration
The amount of a substance that is present in a specified volume
Amount of a substance can be expressed as mass (g or mg), moles (or millimoles), or equivalents (milliequivalents)
Volume is usually expressed as L, dL, or mL
Percent concentration refers to a number of parts in 100 parts of a solution
May be used to express concentration in terms of weight per unit weight, weight per unit volume, or volume per unit volume
Cation
Atom or molecule with a positive charge
Monovalent
One positive or negative charge
Divalent
Two positive or negative charges
Anion
An atom or molecule with a negative charge
Valence
Ions in body fluids combine according to ionic charge (valence) rather than weight
The number of cations in a solution always equals the number of anions to maintain electroneutrality
Useful to express concentrations of solutes in body fluids in equivalents per liter (Eq/L) or milliequivalents per liter (mEq/L) to reflect the charge or valence of the solute
The equivalent weight of a substance is the atomic, molecular, or formula weight of a substance divided by the valence
Electrochemical Equivalence
One equivalent is defined as the weight in grams of an element that combines with or replaces 1 g of H+
Because 1 g of H+ is equal to 1 mol of H+, 1 mol of any univalent anion will combine with this H+ and is equal to 1 equivalent (Eq)
Equivalent Weight
The atomic, molecular, or formula weight divided by the valence
Millimolecular weight/valence = milliequivalent weight
Millimoles x valence = milliequivalents (mEq)
mEq/L = mmol/L x valence
mEq/L = (mg/dL x 10/molecular weight) x valence
How many particles does 1 mol of any substance contain?
Regardless of its weight, 1 mol of any substance contains the same number of particles (6.023 x 1023; Avogadro's number)
What does the osmotic effect of solutes in a solution depend on?
Solutes exert an osmotic effect in solution that is dependent only on the number of particles in solution, not their chemical formula, weight, size, or valence
What is the definition of 1 osmole?
1 g molecular weight of any nondissociable substance so each osmole contains 6.023 x 1023 molecules
If a substance does not dissociate in solution, how many osmoles are there?
1 mol equals 1 Osm
If a substance dissociates in solution, how many osmoles are there?
The number of osmoles equals the number of dissociated particles
Osmolality
The number of osmoles per kg of solvent
Osmolarity
The number of osmoles per liter of solution
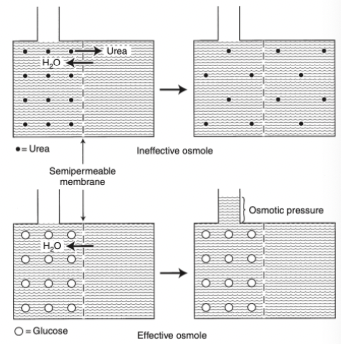
Effective and Ineffective Osmoles
In any fluid compartment, the osmotic effect of a solute is in part dependent on the permeability of the solute across the membranes separating the compartment
Membrane dividing the two compartments is freely permeable to urea and water but is impermeable to glucose
When urea is added to the left compartment, it moves down its concentration gradient from left to right and water moves down the concentration gradient from right to left until there are equal concentrations of urea and water on both sides of the membrane
No fluid rises in the column attached to the left fluid compartment because urea is an ineffective osmole and does not generate osmotic pressure
In biologic fluids, urea is a small molecule that freely diffuses across most cell membranes and therefore does not contribute to effective osmolality
When glucose is added to the left compartment, water moves down its concentration gradient from right to left, but glucose cannot move across the membrane
Osmosis - movement of water from a solution of lesser solute concentration across a semipermeable membrane to a solution of greater solute concentration
The influx of water into the left compartment resulting from the osmotic effect of glucose causes the solution to rise in the column
The height of fluid in the column is proportional to the osmotic pressure generated by glucose
Glucose is an effective osmole because it generates oncotic pressure by causing a shift of water across the boundary membrane
Glucose is an effective osmole in this setting because the boundary membrane is impermeable to glucose, but permeable to water
Tonicity
Effective osmolality of a solution
Measured osmolality of a solution includes both effective and ineffective osmoles
Tonicity of a solution may be less than the measured osmolality if both effective and ineffective osmoles are present
Measured Osmolality
Osmolality determined with an osmometer
Typically not the same as the calculated osmolality estimated using formulas
Calculated Osmolality
Estimate of serum osmolality using various formulas
Often less than measured osmolality because the formulas either exclude some osmotically active particles or estimate their contribution
Osmolal Gap
Difference between measured osmolality and calculated osmolality
Colloids
Large molecular weight (MW = 30,000) particles present in a solution