Dibartola Ch 13; Fielding Ch 10, 11, 13; Acid Base MDRs
1/256
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
257 Terms
What are the main goals of acid-base assessment?
Identify and quantify the magnitude of an acid-base disturbance
Determine the mechanism for the acid-base disturbance by identifying changes in variables that independently alter acid-base balance
Independent Variable
Influence a system from the outside and cannot be affected by changes within the system or by changes in other independent variables
Dependent Variables
Influenced directly and predictably by changes in the independent variables
What is plasma pH determined by in Stewart’s acid-base physiology?
PO2, SID, and [Atot]
![<p>PO2, SID, and [Atot]</p>](https://knowt-user-attachments.s3.amazonaws.com/21d757ff-a93e-4b1a-bd56-719b6e129325.png)
What changes PCO2 in acid-base?
Alveolar ventilation
Has a profound effect on [HCO3-] and pH
PaCO2 is inversely proportional to the alveolar ventilation
What are the two main types of simple ions in plasma?
Nonbuffer ions (strong ions or strong electrolytes)
Buffer ions
Characteristics of Strong Ions
Fully dissociated at physiologic pH and therefore exert no buffering effect
Do exert an electrical effect because the sum of completely dissociated cations does not equal the sum of completely dissociated anions
Difference is the SID
What are the most important strong ions in plasma?
Na+, K+, Ca2+, Mg2+, Cl-, lactate, B-hydroxybutyrate, acetoacetate, and SO42-
What do changes in SID of a magnitude capable of altering acid-base balance usually occur as a result of?
Increasing concentrations of Na+. Cl-, SO42-, or organic anions or decreasing concentrations of Na+ or Cl
What causes a strong ion (metabolic) alkalosis?
An increase in SID (by decreasing [Cl-] or increasing [Na+])
What will cause a strong ion (metabolic) acidosis?
A decrease in SID (by decreasing [Na+ or increasing [Cl-], [SO42-], or organic anions)
Normal Gamblegram
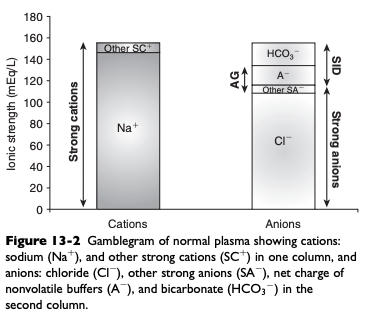
Characteristics of Buffer Ions
Buffer ions are derived from plasma weak acids and bases that are not fully dissociated at physiologic pH
Conventional dissociation reaction for a weak acid (HA) and its conjugated base (A-): HA <-> H+ + A-
For a weak acid to act as an effective buffer, its pKa (negative logarithm of the weak acid dissociation constant Ka) lies within the range of pH +/- 1.5
Normal plasma pH is approximately 7.4 so substances with a pKa between 5.9 and 8.9 can act as buffers
What are the main nonvolatile plasma buffers?
The main nonvolatile plasma buffers act as weak acids at physiologic pH (e.g. phosphate, imidazole [histidine] groups on plasma proteins)
Non-HCO3- buffer system
Form a closed system containing a fixed quantity of buffer
What is Atot the sum of?
A in dissociated [A-} and undissociated [HA] forms
What are the six primary acid base derangements that result from the strong ion approach?
Respiratory alkalosis
Strong ion alkalosis
Nonvolatile buffer ion alkalosis
Respiratory acidosis
Strong ion acidosis
Nonvolatile buffer ion acidosis
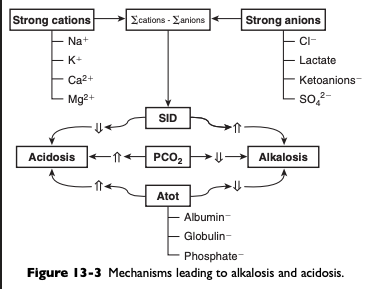
What causes respiratory acidosis?
Increases in PCO2
What causes respiratory alkalosis?
Decreases in PCO2
What are the major contributors to [Atot]
Albumin, globulins, and inorganic phosphate
What do changes in albumin, globulins, and inorganic phosphate occur as a result of?
Changes in plasma albumin, phosphate, or globulin concentrations can occur in response to a change in the distribution volume for the three factors
Changes can occur due to increases or decreases in the total number or moles in plasma or extracellular fluid with no change in the distribution volume
What causes nonvolatile buffer ion alkalosis?
Hypoalbuminemia
Nonvolatile Buffer Ion Alkalosis - Hypoalbuminemia
Phosphate is quantitatively the second most important component of [Atot] and normally is present in plasma at a low concentration so hypophosphatemia is not a clinically important cause of nonvolatile buffer ion (metabolic) alkalosis
Globulin is quantitatively the third most important component of [Atot] but decreases in plasma globulin concentration usually accompany decreases in plasma albumin concentration
Nonvolatile buffer ion alkalosis in dogs and cats is usually due to hypoalbuminemia
The increase in pH secondary to hypoalbuminemia should result in ventilatory compensation (hypoventilation) or a decrease in SID
Presence of hypoalbuminemia complicates identification of increased unmeasured anions (lactate, ketoanions) because hypoproteinemia increases pH but also decreases AG
Calculation of the SIG is preferred in dogs and cats with hypoalbuminemia because hypoproteinemia will not alter the SIG
Treatment for hypoalbuminemic alkalosis should be directed at the underlying cause and the decreased colloid oncotic pressure
![<ul><li><p><span>Phosphate is quantitatively the second most important component of [Atot] and normally is present in plasma at a low concentration so hypophosphatemia is not a clinically important cause of nonvolatile buffer ion (metabolic) alkalosis</span></p></li><li><p><span>Globulin is quantitatively the third most important component of [Atot] but decreases in plasma globulin concentration usually accompany decreases in plasma albumin concentration</span></p></li><li><p><span>Nonvolatile buffer ion alkalosis in dogs and cats is usually due to hypoalbuminemia</span></p></li><li><p>The increase in pH secondary to hypoalbuminemia should result in ventilatory compensation (hypoventilation) or a decrease in SID</p></li><li><p><span>Presence of hypoalbuminemia complicates identification of increased unmeasured anions (lactate, ketoanions) because hypoproteinemia increases pH but also decreases AG</span></p><ul><li><p><span>Calculation of the SIG is preferred in dogs and cats with hypoalbuminemia because hypoproteinemia will not alter the SIG</span></p></li></ul></li><li><p><span>Treatment for hypoalbuminemic alkalosis should be directed at the underlying cause and the decreased colloid oncotic pressure</span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/0233e569-c887-43e8-abe2-a9bc64f2a098.png)
What causes nonvolatile buffer ion acidosis?
Hyperphosphatemia
Nonvolatile Buffer Ion Acidosis - Hyperphosphatemia
Hyperphosphatemia can cause a large increase in [Atot] leading to metabolic acidosis
Hyperalbuminemia is rare but can lead to metabolic acidosis
Most important cause of hyperphosphatemic acidosis is renal failure
Treatment for hyperphosphatemic acidosis should be directed at the underlying cause
Sodium bicarbonate administered IV may be used as an adjunctive therapy in patients with hyperphosphatemic acidosis because the increase in plasma sodium concentration results in an increase in plasma SID, expansion of the extracellular fluid volume, and increased urine production
This decreases plasma phosphorous concentration
![<ul><li><p><span>Hyperphosphatemia can cause a large increase in [Atot] leading to metabolic acidosis</span></p></li><li><p><span>Hyperalbuminemia is rare but can lead to metabolic acidosis</span></p></li><li><p><span>Most important cause of hyperphosphatemic acidosis is renal failure</span></p></li><li><p><span>Treatment for hyperphosphatemic acidosis should be directed at the underlying cause</span></p><ul><li><p><span>Sodium bicarbonate administered IV may be used as an adjunctive therapy in patients with hyperphosphatemic acidosis because the increase in plasma sodium concentration results in an increase in plasma SID, expansion of the extracellular fluid volume, and increased urine production</span></p><ul><li><p><span>This decreases plasma phosphorous concentration</span></p></li></ul></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/91c07549-3494-4361-9df8-bd666fae16fc.png)
What causes changes in SID?
Changes in [HCO3-], BE, [Na+], or [Cl-] from their reference values
What causes a strong ion (metabolic) acidosis?
Decrease in SID
What causes a strong ion (metabolic) alkalosis?
An increase in SID
What are the three general mechanisms by which SID can change?
Increase in strong anions relative to strong cations
Decrease in strong anions relative to strong cations
A change in the free water content of plasma (change in the extracellular fluid volume) with no change in strong anions relative to strong cations
What are the three general mechanisms by which SID can increase leading to metabolic alkalosis?
An increase in [Na+]
A decrease in [Cl-]
A decrease in free plasma water
Concentration Alkalosis
Develops whenever a deficit of free water in plasma occurs
Recognized clinically by the presence of hypernatremia or hyperalbuminemia
Solely decreasing the content of water increases the plasma concentration of all strong cations and strong anions and thus increases SID
Decrease in water content increases Atot but the increase in SID has a greater effect on pH
Therapy for concentration alkalosis should be directed at treating the underlying cause responsible for the change in [Na+]
![<ul><li><p>Develops whenever a deficit of free water in plasma occurs</p><ul><li><p>Recognized clinically by the presence of hypernatremia or hyperalbuminemia</p></li><li><p>Solely decreasing the content of water increases the plasma concentration of all strong cations and strong anions and thus increases SID</p><ul><li><p>Decrease in water content increases Atot but the increase in SID has a greater effect on pH</p></li></ul></li></ul></li><li><p>Therapy for concentration alkalosis should be directed at treating the underlying cause responsible for the change in [Na+]</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/3b723d97-24af-4de7-ad37-2cc5cbfdfdf8.png)
Why will a decrease in ECF volume alone not change acid-base status?
A decrease in ECF volume alone will not alter acid-base status because such a decrease in volume does not change any of the independent variables and therefore cannot change acid-base status
If the decrease in ECF volume is associated with a relatively greater loss of free water, then an acid-base change (concentration alkalosis) will result because of the increase in SID
Hypochloremic Alkalosis
If there is no change in the water content of plasma, plasma [Na+] will be normal
When water content is normal, SID changes only as a result of changes in strong anions
If [Na+] remains constant, decreases in [Cl-] can increase SID (hypochloremic alkalosis)
May be caused by an excessive loss of chloride relative to sodium or by administration of substance containing more sodium than chloride as compared with normal ECF composition
Excessive loss of chloride relative to sodium as compared with normal ECF composition can occur in the urine after administration of diuretics that cause chloride wasting (e.g. furosemide) or when the fluid lost has a low or negative SID (vomiting)
![<ul><li><p><span>If there is no change in the water content of plasma, plasma [Na+] will be normal</span></p><ul><li><p><span>When water content is normal, SID changes only as a result of changes in strong anions</span></p></li><li><p><span>If [Na+] remains constant, decreases in [Cl-] can increase SID (hypochloremic alkalosis)</span></p></li></ul></li><li><p><span>May be caused by an excessive loss of chloride relative to sodium or by administration of substance containing more sodium than chloride as compared with normal ECF composition</span></p><ul><li><p><span>Excessive loss of chloride relative to sodium as compared with normal ECF composition can occur in the urine after administration of diuretics that cause chloride wasting (e.g. furosemide) or when the fluid lost has a low or negative SID (vomiting)</span></p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/505f61f0-d6c6-4d9e-a1b9-87ebe138efc0.png)
Chloride Resistant Metabolic Alkalosis
Does not respond to chloride administration alone
Usually caused by hyperadrenocorticism or hyperaldosteronism
What are the three general mechanisms that can cause SID to decrease resulting in SID (metabolic) acidosis?
A decrease in [Na+]
An increase in [Cl-]
An increased concentration of other strong anions (e.g. L-lactate, B-hydroxybutyrate, sulfate)
Dilutional Acidosis
Occurs whenever there is an excess of water in plasma
Recognized clinically by the presence of hyponatremia
Increasing the water content of plasma decreases the concentration of all strong cations and strong anions, and thus SID
An increase in water content also decreases Atot, but the decrease in SID has a greater effect on pH
An increase in ECF volume alone will not alter acid-base status because such an increase in volume does not change any of the independent variables
If the increase in ECF volume is associated with a relatively greater addition of free water, then an acid-base change (dilutional acidosis) will result primarily because of the decrease in SID
Large increases in free water are necessary to cause an appreciable decrease in SID
Associated with congestive heart failure, hypoadrenocorticism, third space loss of sodium, and hypotonic fluid administration
Therapy for dilutional acidosis should be directed at the underlying cause of the change in [Na+]
![<ul><li><p><span>Occurs whenever there is an excess of water in plasma</span></p><ul><li><p><span>Recognized clinically by the presence of hyponatremia</span></p></li><li><p><span>Increasing the water content of plasma decreases the concentration of all strong cations and strong anions, and thus SID</span></p></li></ul></li><li><p><span>An increase in water content also decreases Atot, but the decrease in SID has a greater effect on pH</span></p></li><li><p><span>An increase in ECF volume alone will not alter acid-base status because such an increase in volume does not change any of the independent variables</span></p><ul><li><p><span>If the increase in ECF volume is associated with a relatively greater addition of free water, then an acid-base change (dilutional acidosis) will result primarily because of the decrease in SID</span></p></li></ul></li><li><p><span>Large increases in free water are necessary to cause an appreciable decrease in SID</span></p></li><li><p><span>Associated with congestive heart failure, hypoadrenocorticism, third space loss of sodium, and hypotonic fluid administration</span></p></li><li><p><span>Therapy for dilutional acidosis should be directed at the underlying cause of the change in [Na+]</span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/c92fc67d-76f9-4195-8a47-09766266227a.png)
Hyperchloremic Acidosis
Increases in [Cl-] can substantially decrease SID, leading to hyperchloremic acidosis
May be caused by chloride retention (early renal failure, renal tubular acidosis), excessive loss of sodium relative to chloride (diarrhea), or by administration of substance containing more chloride than sodium as compared with normal ECF composition
Administration of 0.9% NaCl is a common cause in hospitalized patients
Treatment should be directed at correction of the underlying disease process
![<ul><li><p>Increases in [Cl-] can substantially decrease SID, leading to hyperchloremic acidosis</p></li><li><p><span>May be caused by chloride retention (early renal failure, renal tubular acidosis), excessive loss of sodium relative to chloride (diarrhea), or by administration of substance containing more chloride than sodium as compared with normal ECF composition</span></p></li><li><p><span>Administration of 0.9% NaCl is a common cause in hospitalized patients</span></p></li><li><p><span>Treatment should be directed at correction of the underlying disease process</span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/b623155f-53a5-422b-a0d3-2b160e9a6fdb.png)
SID Acidosis Caused by an Increase in Unmeasured Strong Anions (Organic Acidosis)
Accumulation of metabolically produced organic anions (L-lactate, acetoacetate, citrate, B-hydroxybutyrate) or addition of exogenous organic anions (e.g. salicylate, glycolate from ethylene glycol poisoning, formate from methanol poisoning) will cause metabolic acidosis because these strong anions decrease SID
Most frequently encountered causes of organic acidosis in dogs and cats are renal failure (uremic acidosis), diabetic ketoacidosis, lactic acidosis, and ethylene glycol toxicity
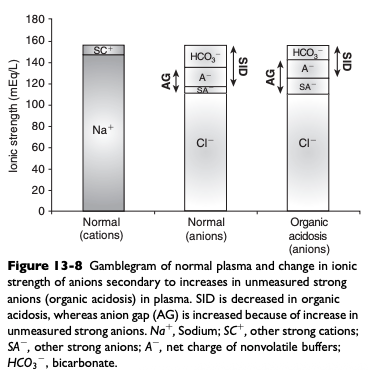
Advantages of the Henderson-Hasselbalch Approach ([HCO3-] or Base Excess and Anion Gap) to Acid Base
Widely and routinely used
Easy to calculate
Disadvantages of Henderson-Hasselbalch Approach ([HCO3-] or Base Excess and Anion Gap) for Acid Base
Descriptive
Anion gap lacks sensitivity and specificity
Does not account for changes caused by protein and phosphorous
Henderson-Hasselbalch Approach ([HCO3-] or Base Excess and Anion Gap) Errors and Limitations
Does not explain effects of temperature on pH
Does not explain dependence on pK1’ on pH
States that there is a linear relationship between pH and log PCO2
Can only accurately be applied to plasma at normal temperature, pH, and protein and sodium concentrations
Advantages of the Strong Ion Model for Acid Base
Mechanistic
Explains effects of protein and phosphorous on pH
Disadvantages of Strong Ion Model for Acid Base
True SID can only be estimated
Algebraic complexity
Errors and Limitations to the Strong Ion Model
Uses hydrogen ion concentration instead of pH
Stewart’s strong ion equation does not algebraically simplify to the Henderson’Hasselbalch equation in an aqueous solution with no proteins
What do clinical signs traditionally used to assess a patient’s fluid deficit evaluate?
Aspects of both hypovolemia and dehydration but provide only a crude estimate of fluid status
Mild Dehydration %
5-8%
5-8% Dehydration Mucous Membranes
Normal to slightly tacky
5-8% Dehydration Capillary Refill Time
Normal (<2s)
5-8% Dehydration Heart Rate
Normal
5-8% Dehydration Other Clinical Signs
Decreased urine production
Moderate Dehydration %
8-10%
8-10% Dehydration Mucous Membranes
Tacky
8-10% Mucous Membranes Capillary Refill Time
Variable (often 2-3 s)
8-10% Dehydration Heart Rate
40-60 bpm
8-10% Dehydration Other Clinical Signs
Decreased arterial blood pressure
Severe Dehydration %
10-12%
10-12% Dehydration Mucous Membranes
Dry
10-12% Dehydration Capillary Refill Time
Variable (often prolonged >4s)
10-12% Dehydration Heart Rate
>60 bpm
10-12% Dehydration Other Clinical Signs
Jugular fill slow; peripheral pulses weak; sunken eyes
Body Weight for Monitoring Fluid Therapy
Sudden increases in body weight might indicate fluid retention - 1 L of fluid weighs approximately 1 kg
Packed Cell Volume and Total Solids Concentration for Monitoring Fluid Therapy
PCV has utility as a prognostic indicator, with higher values associated with a poorer prognosis
PCV and TS are not always as useful in directing fluid therapy in horses as they are in other species
In adult horses, there is great individual variation in PCV, and PCV is often increased in distressed or excited animals as a result of splenic contraction
Presence of anemia will obscure the increase in PCV expected with hypovolemia
Many horses that require fluid resuscitation also experience protein loss
An increase in both PCV and TS (or total protein or albumin) concentration is consistent with a loss of plasma volume
Causes of Hyperlactatemia in Large Animals
A decrease in tissue perfusion and oxygen delivery (DO2) with subsequent anaerobic metabolism is the most important cause of hyperlactatemia in large animals
Hyperlactatemia will occasionally occur in the face of apparently adequate DO2 usually in association with intense systemic inflammation
Horses with advanced liver failure might have an increase in blood lactate concentration as a result of impaired metabolism
What does venous lactate measured in the peripheral veins reflect?
Venous lactate concentrations measured in peripheral veins reflect the adequacy of global perfusion; measured venous lactate concentrations can be normal despite significant perfusion deficits of individual tissue beds
What happens if blood samples for lactate measurement are stored?
Unless samples are collected into tubes containing sodium fluoride, erythrocyte lactate production will continue and can affected measured concentrations in samples stored for longer than 30-60 minutes
Anion Gap as an Estimate of Lactate Concentration
Historically, the anion gap (AG) has been used as an estimate of lactate concentration, but should be interpreted with caution as it can be increased by anions other than lactate (e.g. azotemia) and is decreased with hypoalbuminemia
An increase in anion gap might also be seen with an increase in D- (bacterial) lactate concentration
Blood Lactate in Neonatal Foals
Blood lactate concentration in normal newborn foals exceeds that in adults for the first 1-3 days of life
Lactate concentrations above the normal age-interpreted ranges for foals, persistent hyperlactatemia, or a very slow decrease in lactate concentration in a neonatal foal should prompt concerns of a fluid deficit
Increased blood lactate concentrations due to hypovolemia and hypoperfusion are expected to decrease to normal over 6-12 hours with effective restoration of vascular volume and tissue perfusion
What is venous oxyhemoglobin saturation (SvO2) dependent on?
Arterial oxyhemoglobin saturation (SaO2), cardiac output, and tissue oxygen demand
A decrease in cardiac output or an increase in tissue oxygen demand will decrease SvO2 as more oxygen is stripped from hemoglobin by the tissues
Causes of Decreased SvO2
Venous oxyhemoglobin saturation is decreased in anemic patients (decreased carrying capacity with a subsequent decrease in arterial oxygen content (CaO2) and in patients with significant pulmonary disease (impaired pulmonary gas exchange with a subsequent decrease in SaO2)
Causes of Increased SvO2
SvO2 can be increased (or normal) in patients with mitochondrial dysfunction or with significant shunting
Where is SvO2 ideally measured from to accurately assess global perfusion?
To accurately assess global perfusion, SvO2 is ideally measured in samples collected from the pulmonary artery
Use of information derived from PA sampling has not consistently produced significant survival benefits in humans and there are concerns related to complications associated with PA catheterization so this procedure is now less commonly performed
Measuring SvO2 from a Central Venous Catheter
Measurement of SvO2 from a central venous catheter (placed in either the cranial or caudal vena cava) provides a good approximation of mixed venous values, is easier to perform, and is considered safer
In neonates, the tip of a standard catheter often lies within the cranial vena cava so central SvO2 monitoring could be performed
Central SvO2 values can also be monitored in horses that have had catheters placed for CVP measurement
SvO2 measured in jugular samples (standard catheters in adult horses) does not reliably reflect central SvO2
What is normal or target SvO2?
Normal or target central SvO2 used in the management of critically ill human patients is 70-75%
In horses with normal pulmonary function and hemoglobin concentrations, values for SvO2 below 70% are suggestive of hypovolemia
Oxygen Extraction Ratio
Oxygen extraction ratio (O2ER) measures the percentage of oxygen removed from the blood as it moves through the tissues
Provides similar information to SvO2 but requires both mixed venous (PA) and arterial samples
Oxygen Extraction Ratio Equations
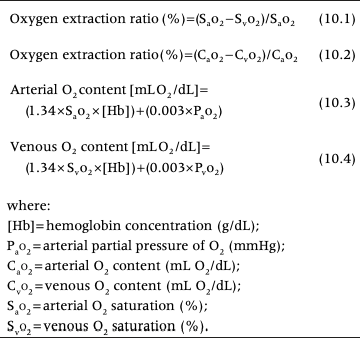
Normal Values for O2ER
Normal values for O2ER are 25-30%
A decrease in cardiac output, an increase in tissue oxygen demand, or anemia increases the O2ER
In hypovolemia, the O2ER is expected to be greater than 25-30% and decrease toward normal as volume is restored and cardiac output improved
What is urine output a marker of?
End-organ (i.e. renal) blood flow
Evaluation of Urine Output for Fluid Therapy Monitoring
Qualitative assessment of urine production is sufficient in many adult horses
In recumbent foals, urine production can be semi-quantitatively measured by weighing urine-soaked absorbent pads
Placement of an indwelling urinary catheter is required to accurately measure urine production but should be considered in hemodynamically unstable patients or those predisposed to decreased renal perfusion
What is the normal range of urine production in adult horses?
15-30 ml/kg/day
What is normal urine output in foals?
Because healthy nursing foals consume large volumes of milk (~8-10 ml/kg/h), they produce large volumes (~4-8 ml/kg/h) of dilute urine
Urine production in foals receiving combinations of IV fluids, parenteral nutrition, and other medications will be highly variable but should be ~2/3 of total fluid inputs if they are meeting requirements
Potential Causes of Decreased Urine Production
Potential causes of decreased urine production might include hypovolemia or hypotension, anuric or oliguric renal disease, and obstruction of the urinary tract or uroperitoneum in foals
What should USG be with hypovolemia and dehydration in the absence of renal disease?
Concentrated (USG >1.035)
What should USG be with adequate resuscitation?
Approximately isosthenuric (1.008-1.012)
Why can using USG for evaluation of fluid therapy be difficult in critically ill patients?
Disease in critically ill patients can interfere with ADH function and other aspects of renal function confounding interpretation of USG
These patients can produce large volume of dilute urine (USG <1.008) in the face of hypovolemia
Central Venous Pressure (CVP)
Pressure within the intrathoracic portion of the vena cava
What does CVP reflect?
The balance between the pumping ability of the heart and venous return
What can CVP be determined by?
Cardiac function, central venous blood volume, and venomotor tone
What can CVP be used to assess?
Right side cardiac function but in large animal patients, CVP measurements are most often used to assess intravascular volume
How do you measure CVP?
Measurement of CVP in horses requires placement of the tip of a fluid filled catheter in the cranial vena cava or right atrium
In neonatal foals, the tips of standard 20 cm IV catheters often lie within the cranial vena cava
Special catheters are made for CVP measurement in adult horses but can also use sterile polyethylene or polypropylene tubing passed through a 10 or 14 gauge venous catheter
CVP catheter is connected to a water manometer for intermittent pressure measurements or to a pressure transducer and monitor for continuous monitoring
Small oscillations in the fluid meniscus (or the pressure reading) synchronized with respiration confirm the intrathoracic location of the catheter tip
Measurements of CVP in standing horses are referenced to the right atrium so the zero mark on a water manometer or the electronic pressure transducer should be placed at the point of the shoulder
It is critical that this positioning is identical between measurements
In recumbent horses, CVP measurements should be zeroed to the sternal manubrium
The horse's head should be held in a neutral position that is constant between measurements and all IV infusions should be stopped during CVP measurement
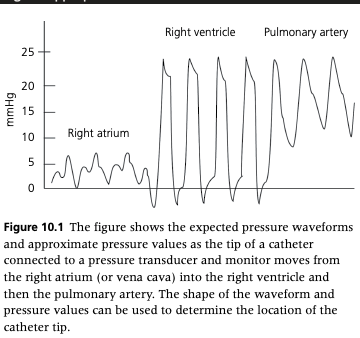
How do you determine the location of the catheter tip for CVP measurement?
Using a pressure transducer and monitor, the location of the catheter tip can be confirmed by observing the appropriate waveform
The location of the catheter tip can also be confirmed using echocardiography
Confirmation of catheters in neonatal foals can be achieved with portable thoracic radiographs
When using a water manometer, you can pass the catheter into the ventricle (recognized by the systolic pressures of approximately 20-34 cmH2O) and then withdraw the catheter so that the tip lies within the right atrium or cranial vena cava as assessed by the decrease in measured pressures
What causes the a-wave on a CVP tracing?
Atrial contraction
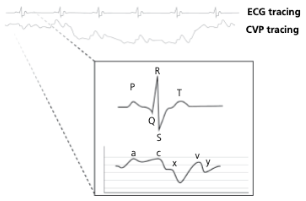
What causes the c-wave on a CVP tracing?
Bulge of tricuspid valve into the atria during ventricular contraction (systole). The c wave is increased in size with tricuspid insufficiency
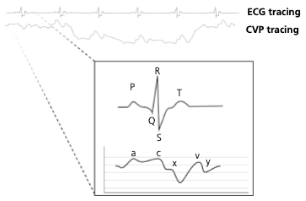
What causes the x-descent on a CVP tracing?
Reduction in atrial pressure during ventricular contraction and ejection
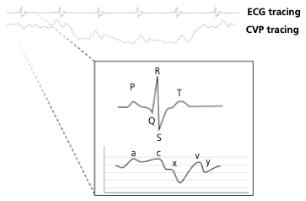
What causes the v-wave on a CVP tracing?
Early atrial filling, while tricuspid valve is closed
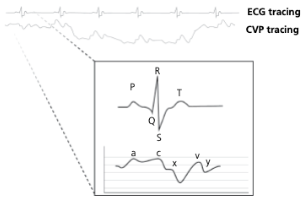
What causes the y-descent on a CVP tracing?
Rapid flow of blood into ventricle from the atria after tricuspid valve opens
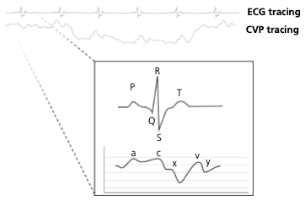
When is CVP greatest and lowest during spontaneous respiration? With mechanical ventilation?
During spontaneous respiration, CVP is greatest during expiration (positive intrathoracic pressure) and lowest within inspiration (negative intrathoracic pressure)
With mechanical ventilation, this relationship is reversed
When should CVP be measured?
End-expiration
The most precise measure of CVP is the mean of the a-wave at end-expiration when using an electronic pressure transducer or monitor
How do water manometers tend to affect CVP readings?
Water manometers tend to dampen the pressure changes so measurements are less precise and they can overestimate CVP by up to 5 cmH2O
What is normal CVP in neonatal foals?
CVP in normal neonatal foals range from 3-12 cmH2O during the first two weeks of life
What is CVP in healthy standing adult horses measured with a water manometer?
Ranges between 5 and 15 cmH2O
How can CVP pressures measured in mmHg be converted to cmH2O?
By multiplying by 1.3