APHY 201 Ch 1, 2: Homeostasis, Chemistry Ivy Tech
1/88
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
89 Terms
explain why chemistry is an important part of the study of physiology
all the cells in your organs are composed of chemicals and chemical reactions are involved in all of your body's movements and cycles
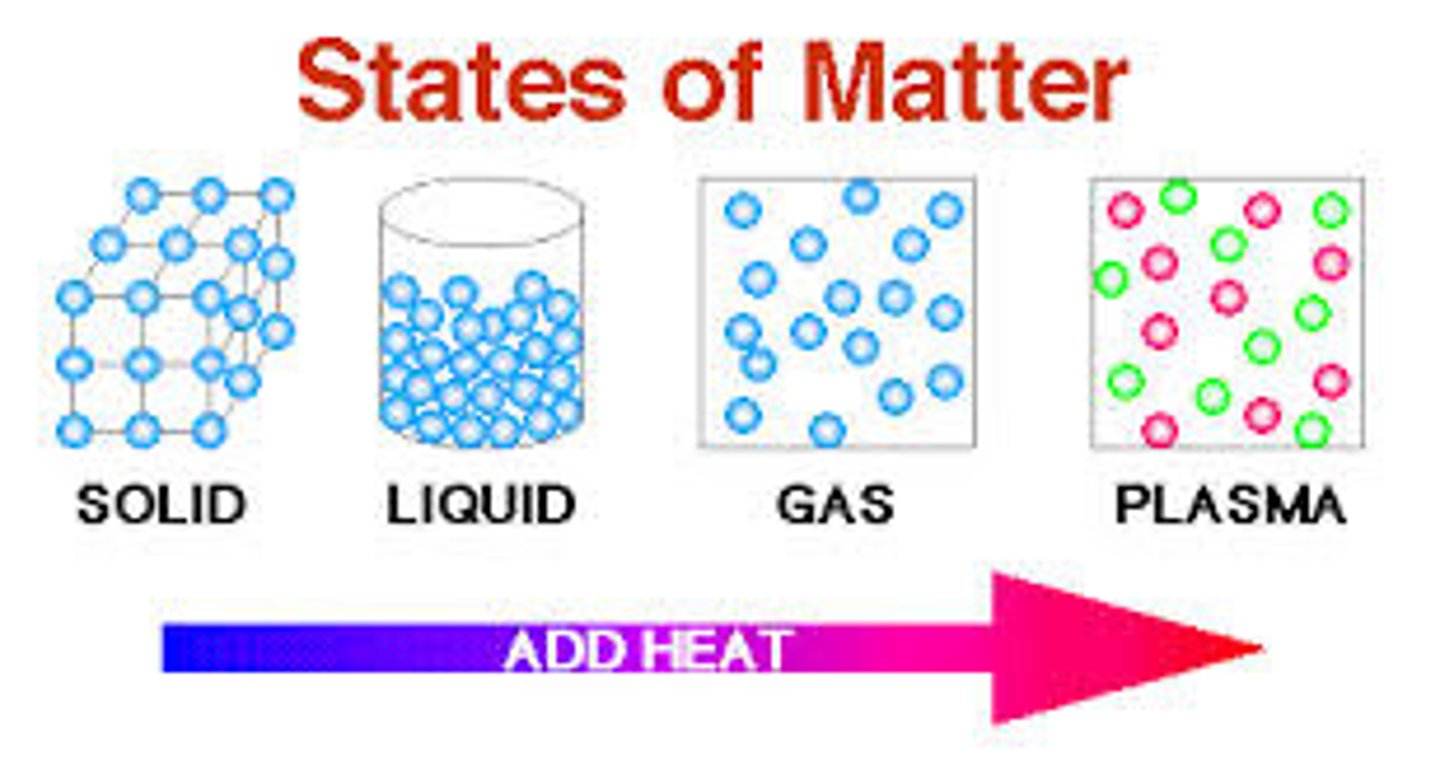
matter
Anything that occupies space and has mass; the physical material of the universe
element
a pure substance that cannot be created or broken down by ordinary chemical means
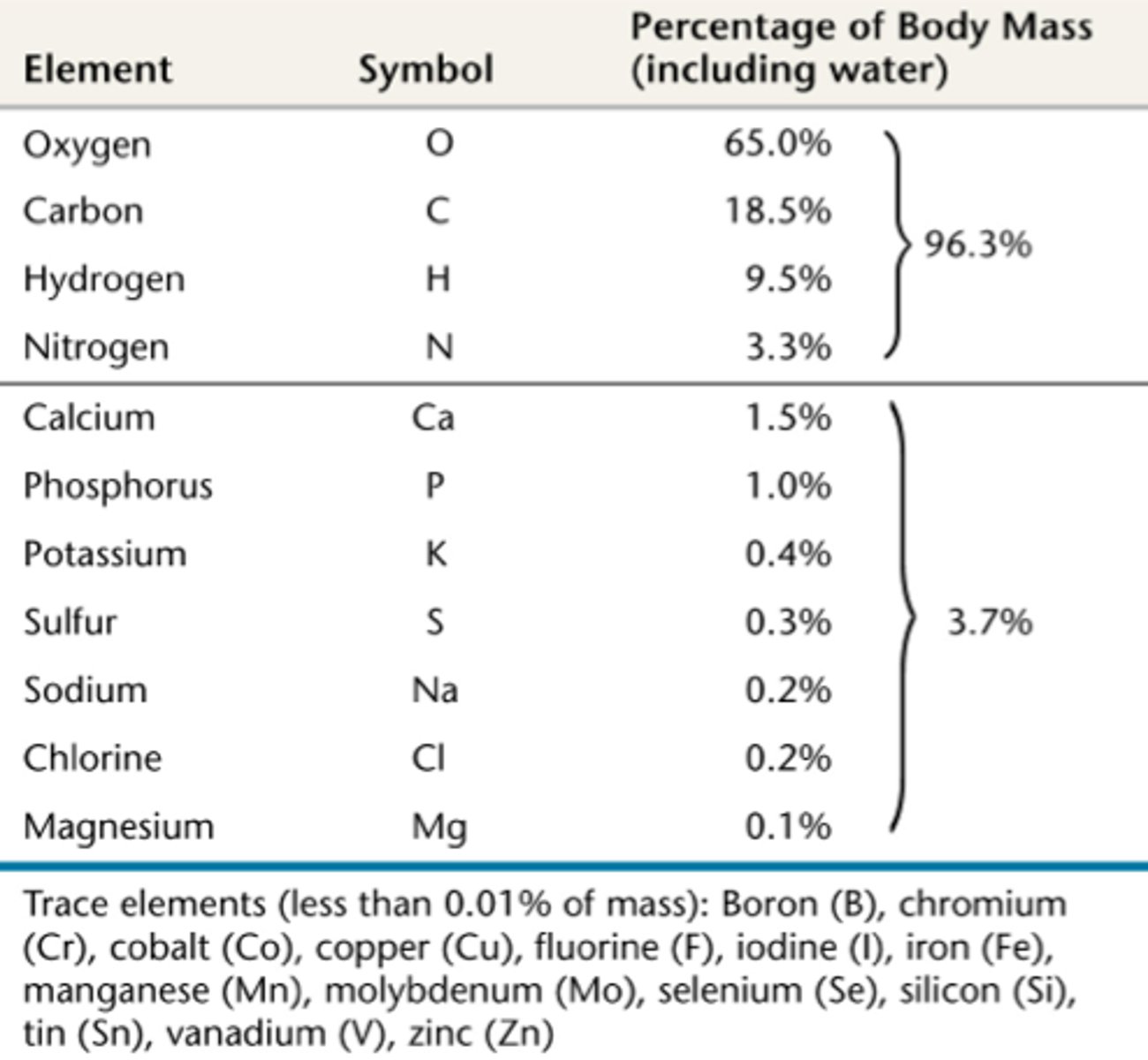
most abundant elements in humans
oxygen (O), carbon (C), hydrogen (H), nitrogen (N)
compound
substance composed of two or more elements joined by chemical bonds
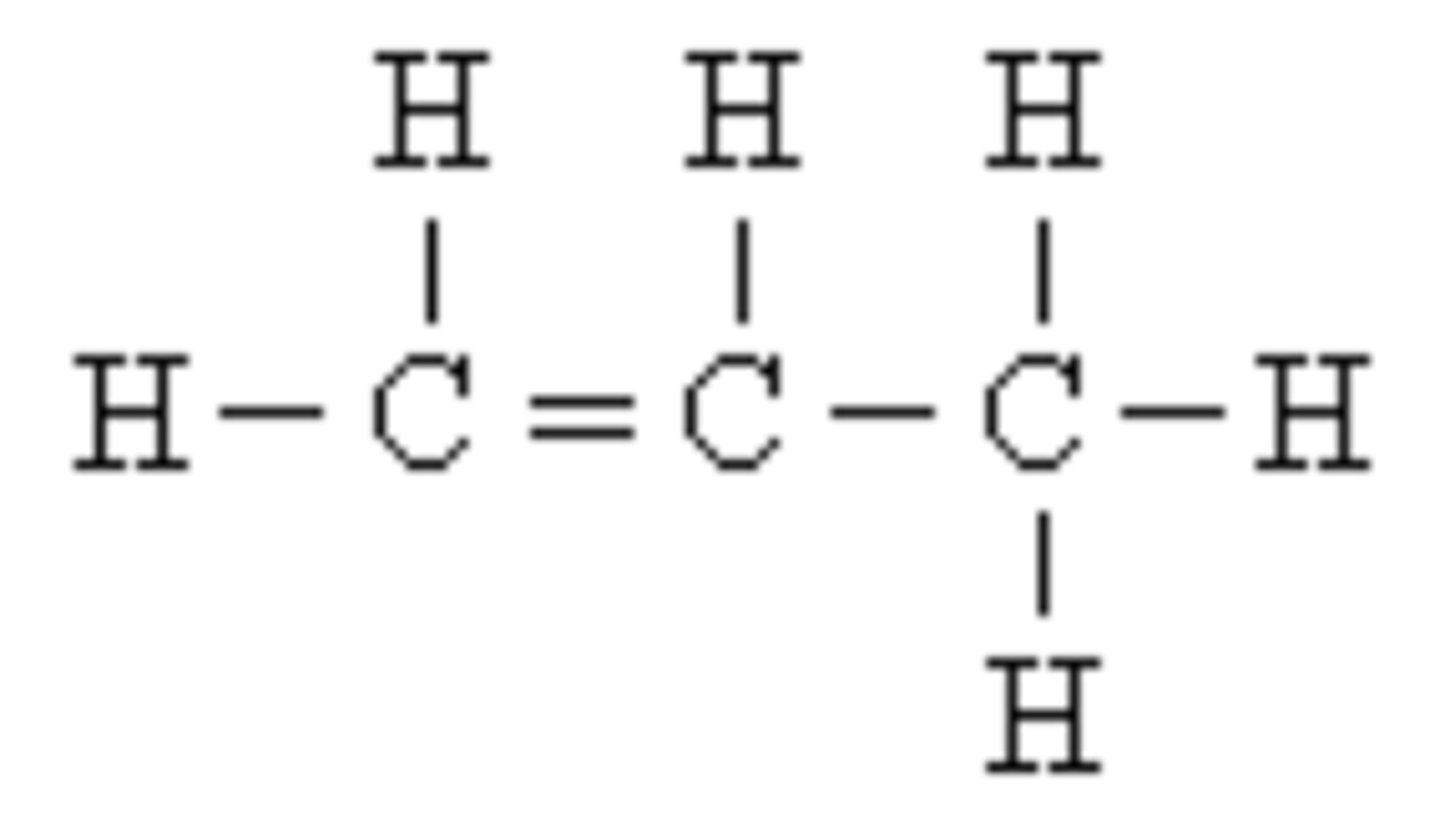
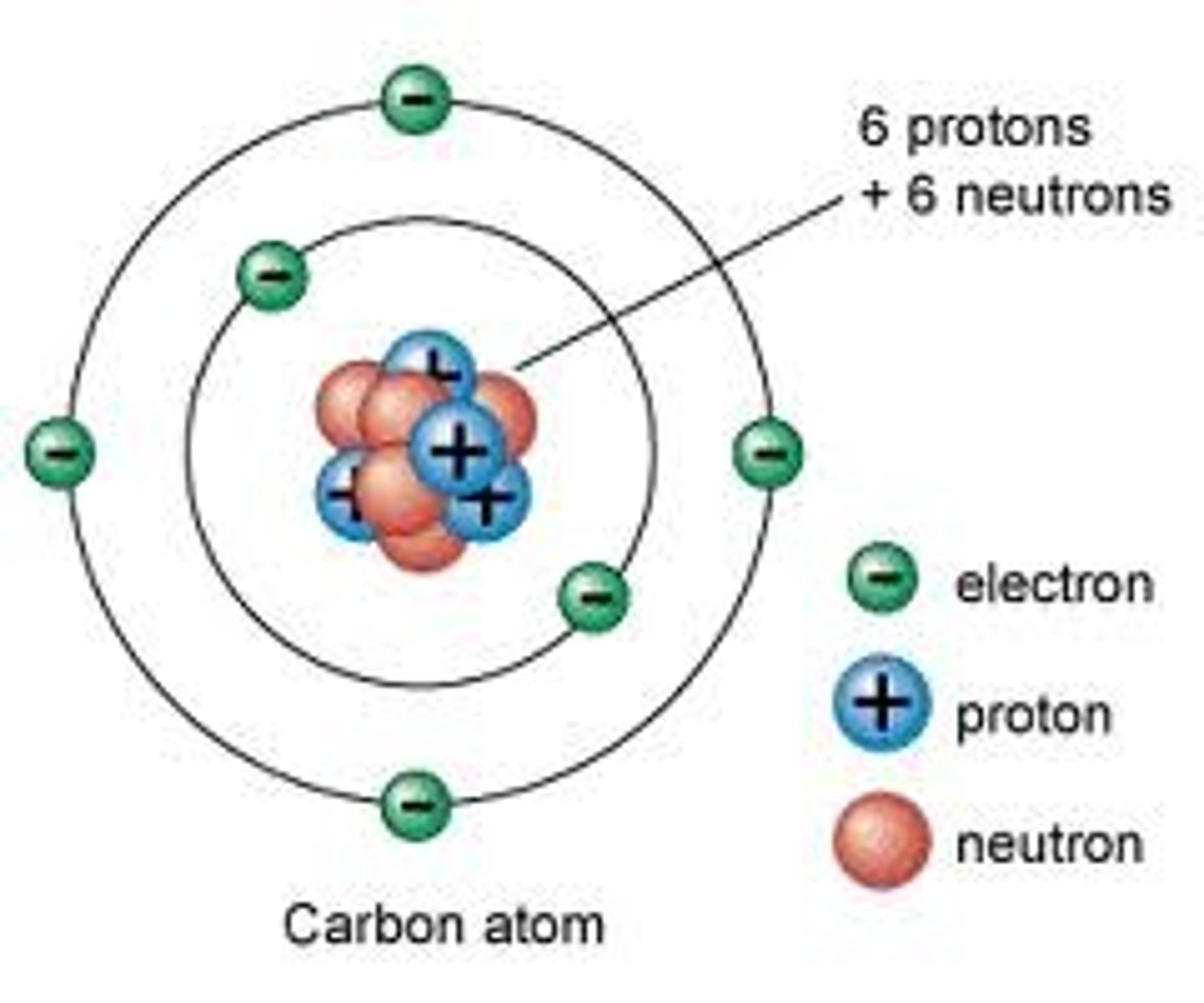
proton
A subatomic particle that has a positive charge and that is found in the nucleus of an atom
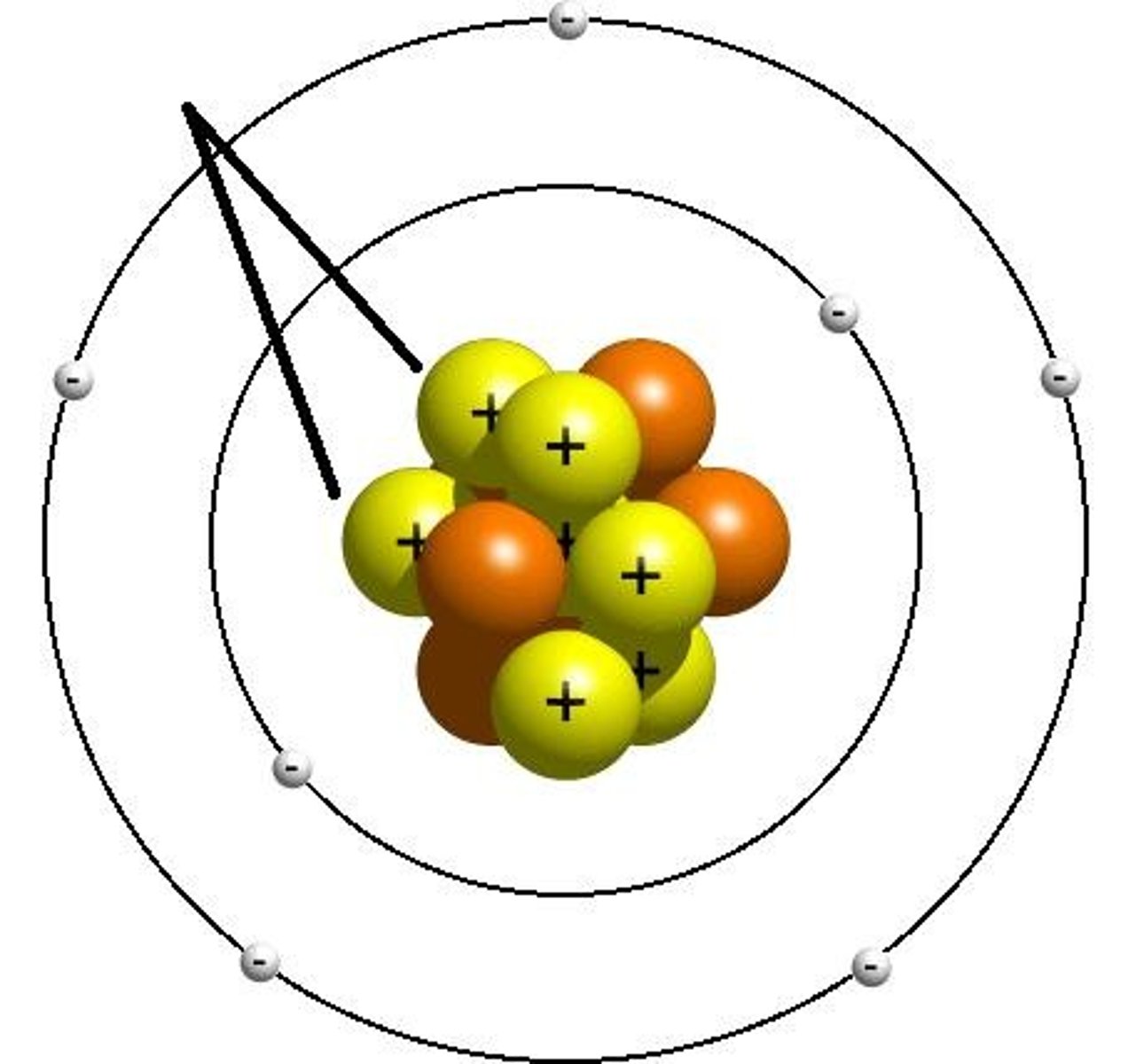
neutron
A subatomic particle that has no charge and that is found in the nucleus of an atom
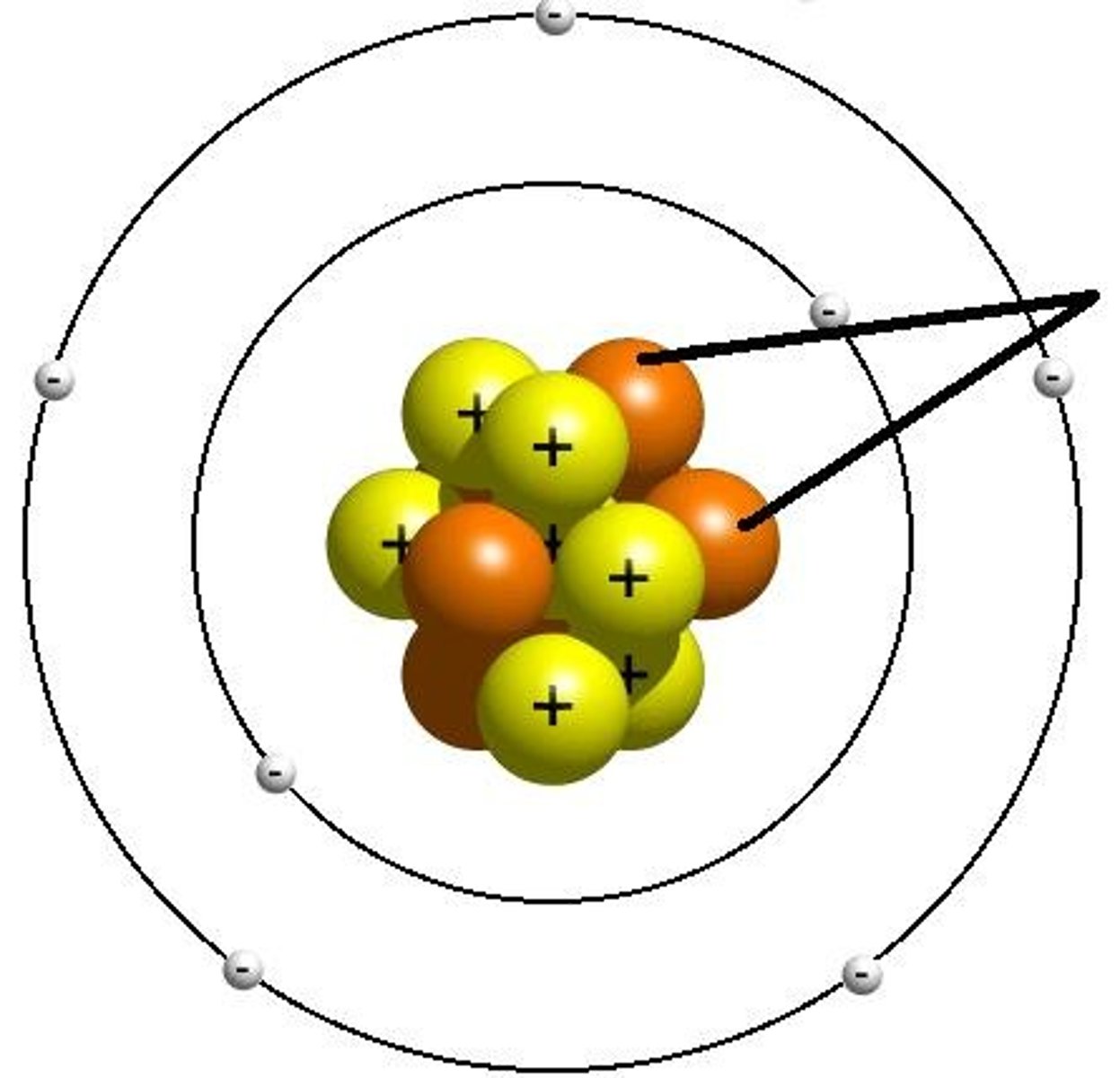
electron
A subatomic particle that has a negative charge and found outside the nucleus
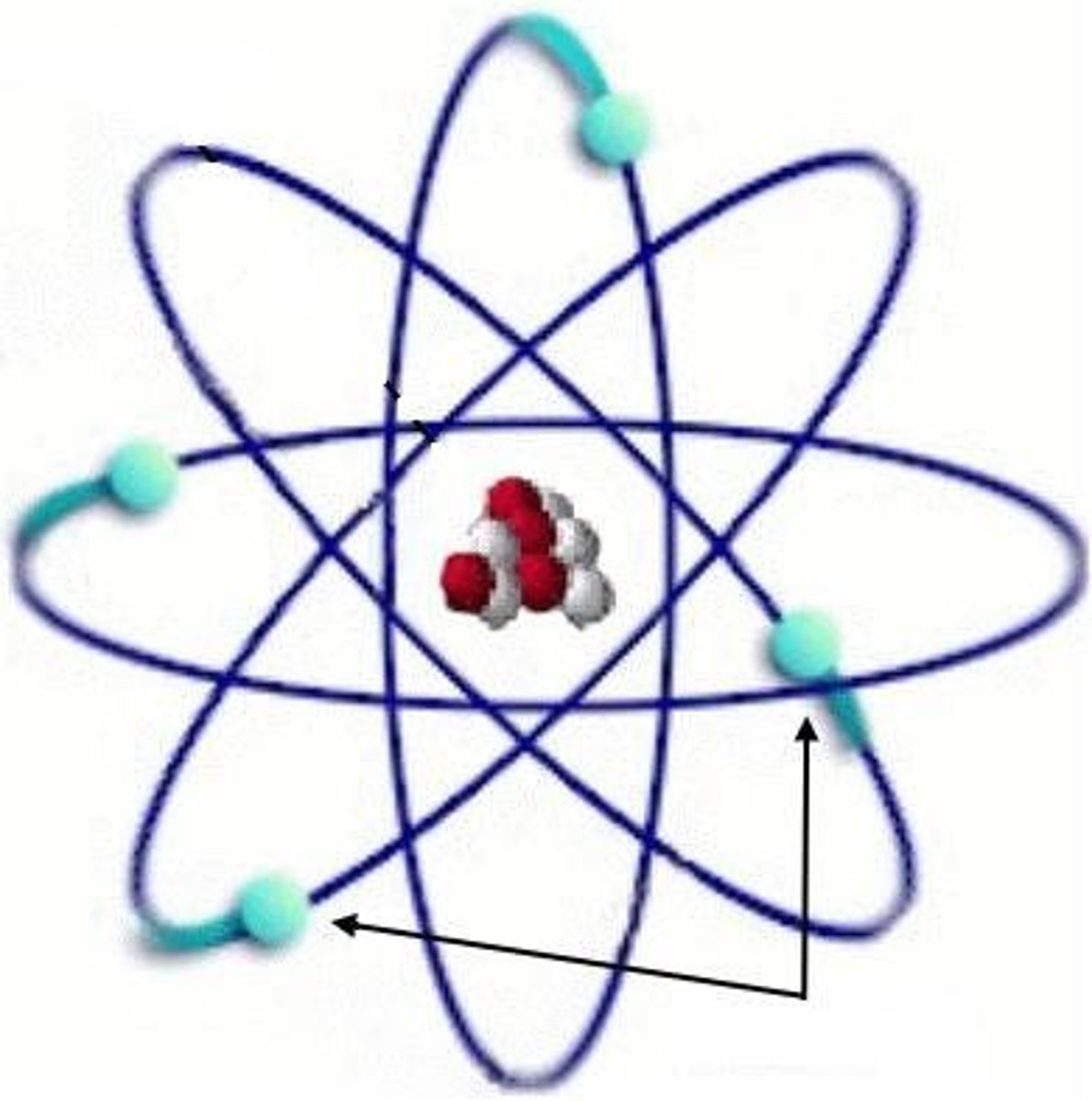
nucleus
center of an atom, contains protons and neutrons
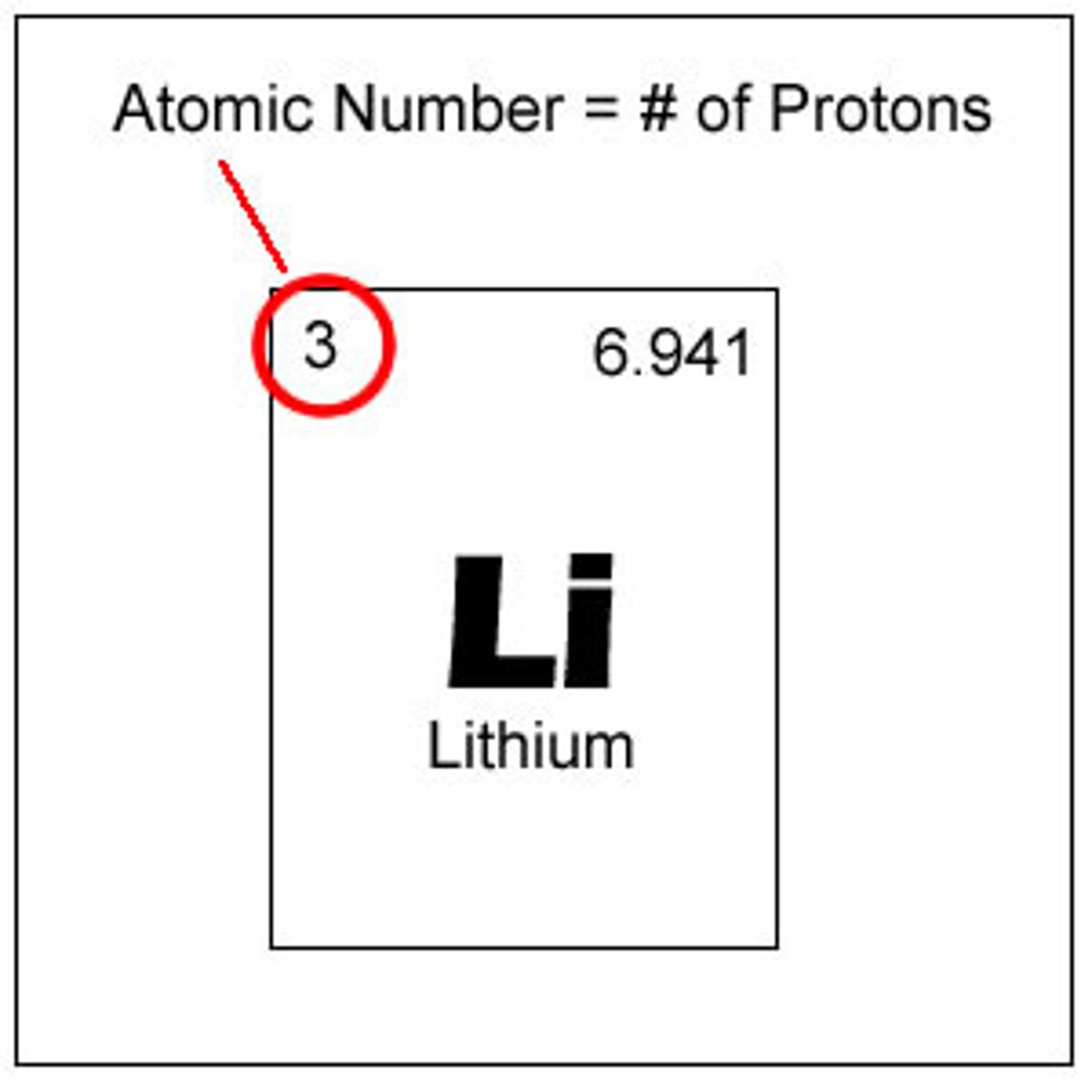
atomic number
the number of protons in the nucleus of an atom, which determines the chemical properties of an element and its place in the periodic table
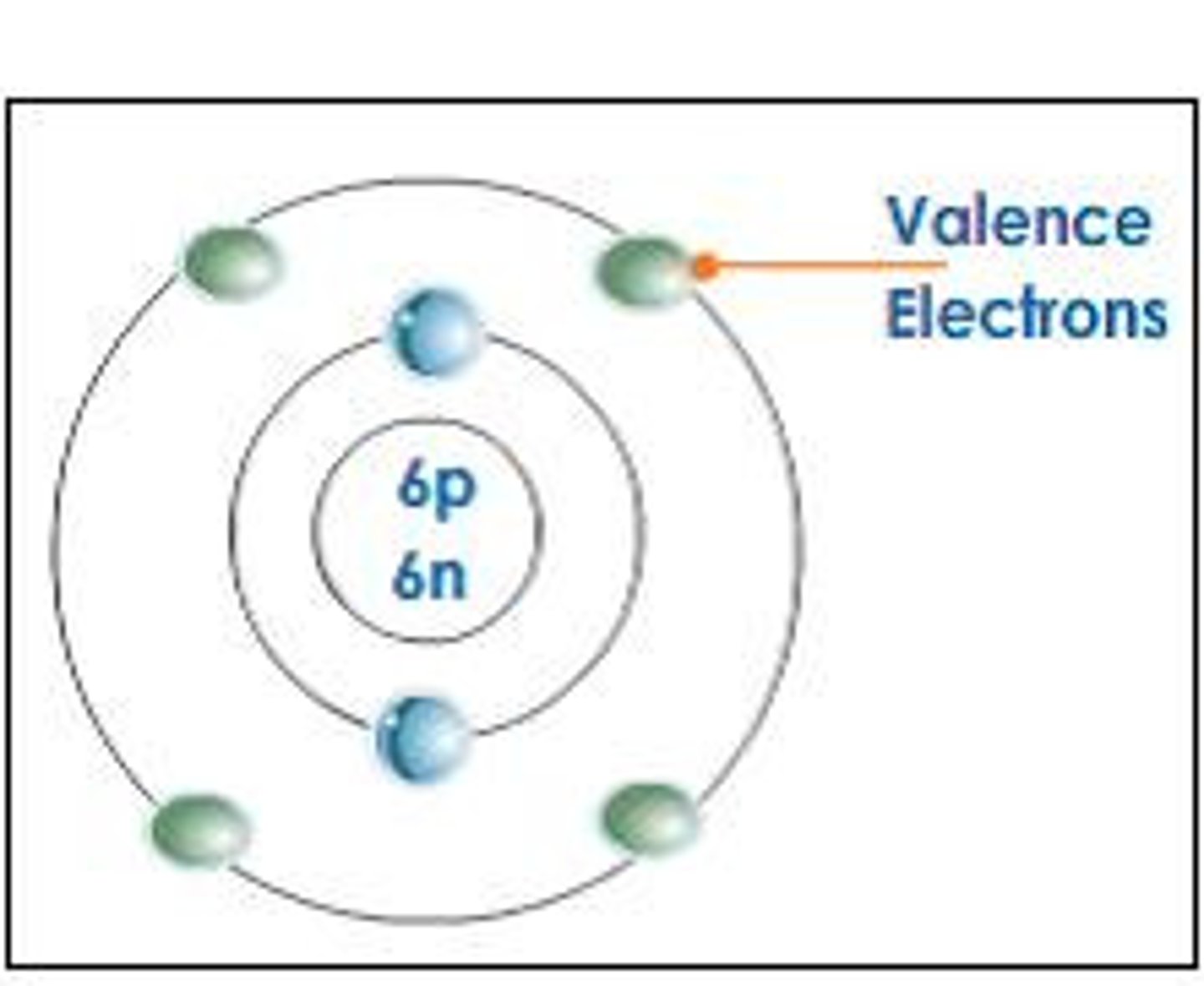
valence electrons
The electrons in the outermost shell (main energy level) of an atom; these are the electrons involved in forming bonds.
bond
a weak or strong electrical attraction that holds atoms in the same vicinity
molecule
atoms bonded together
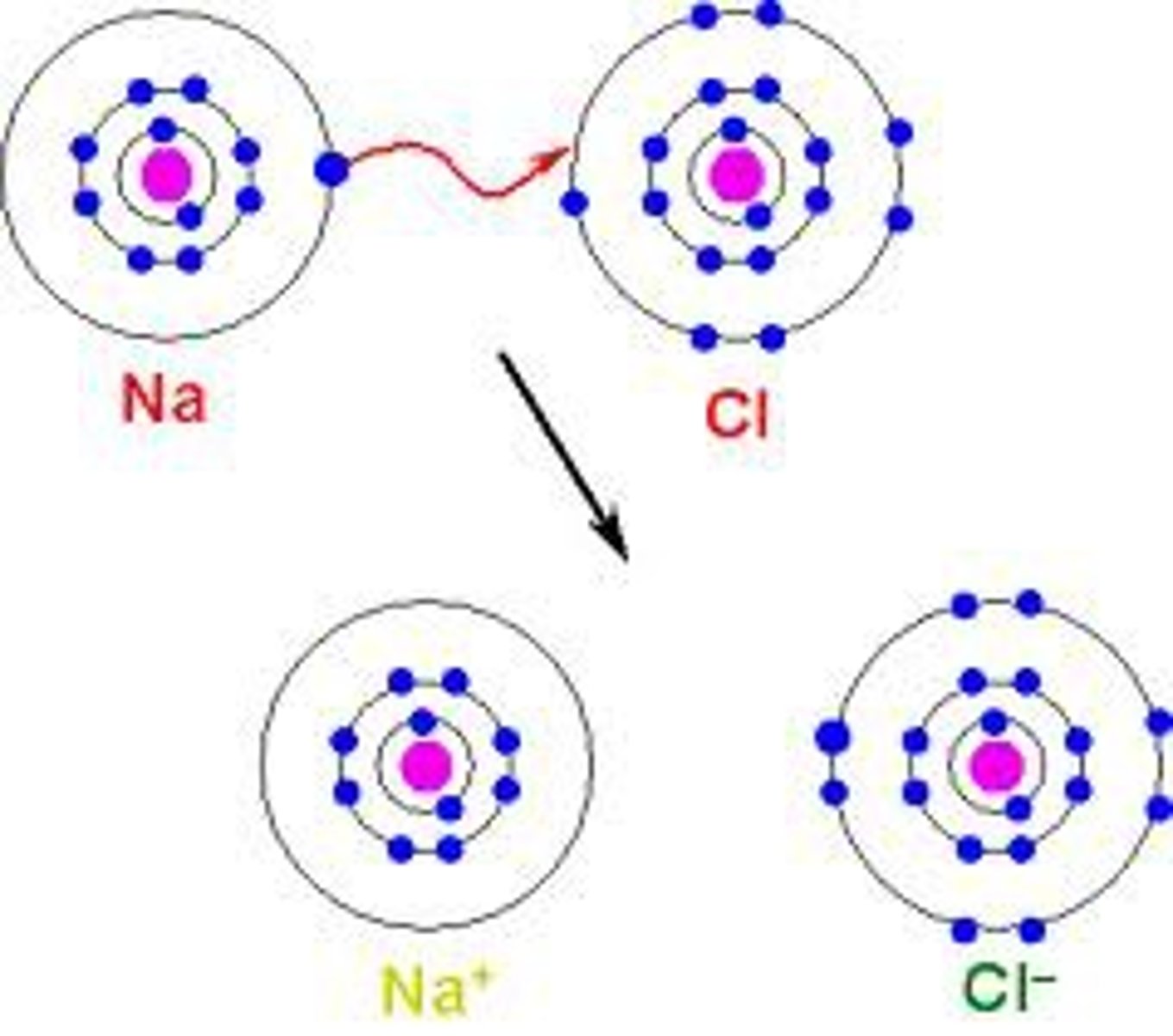
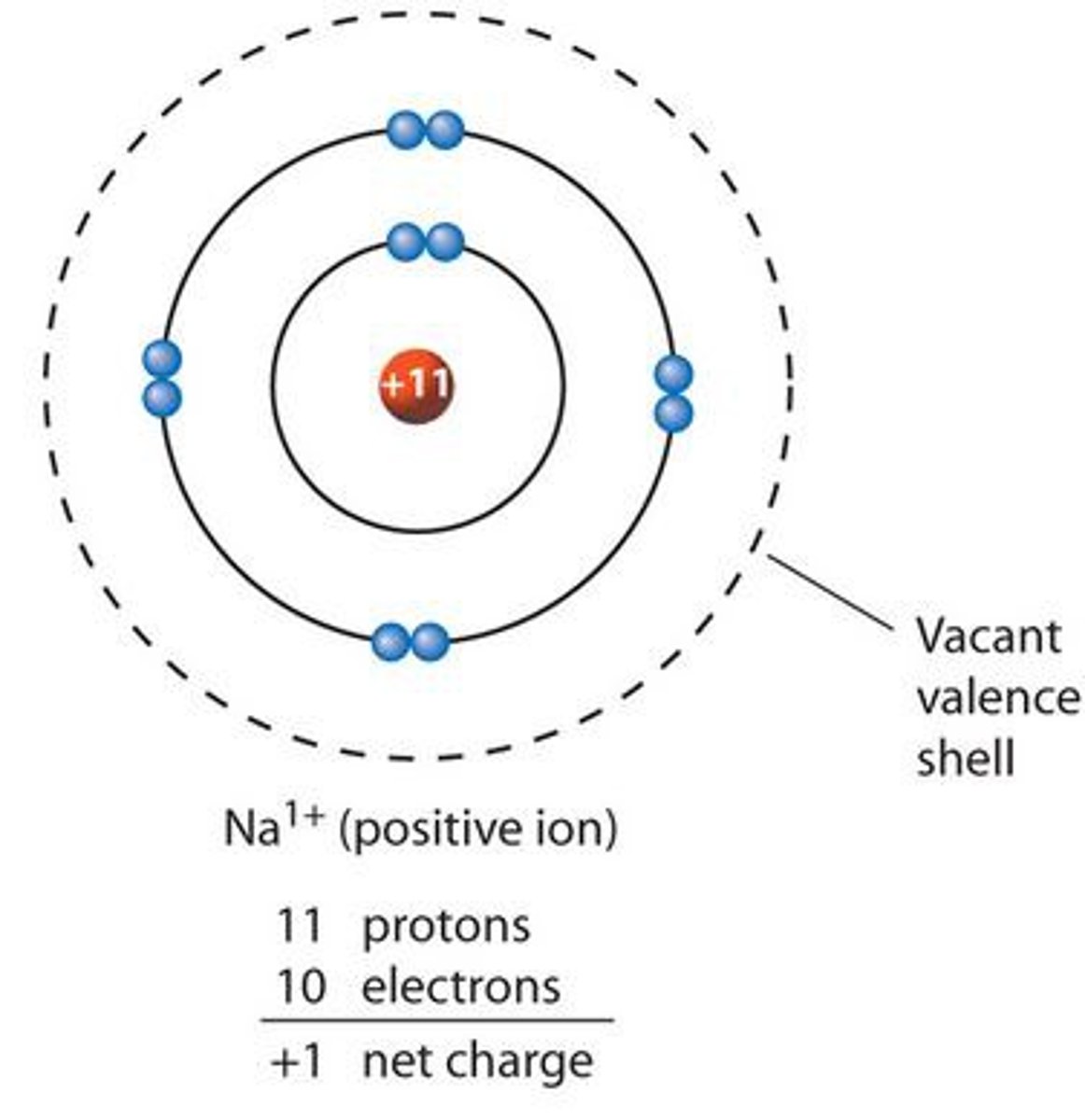
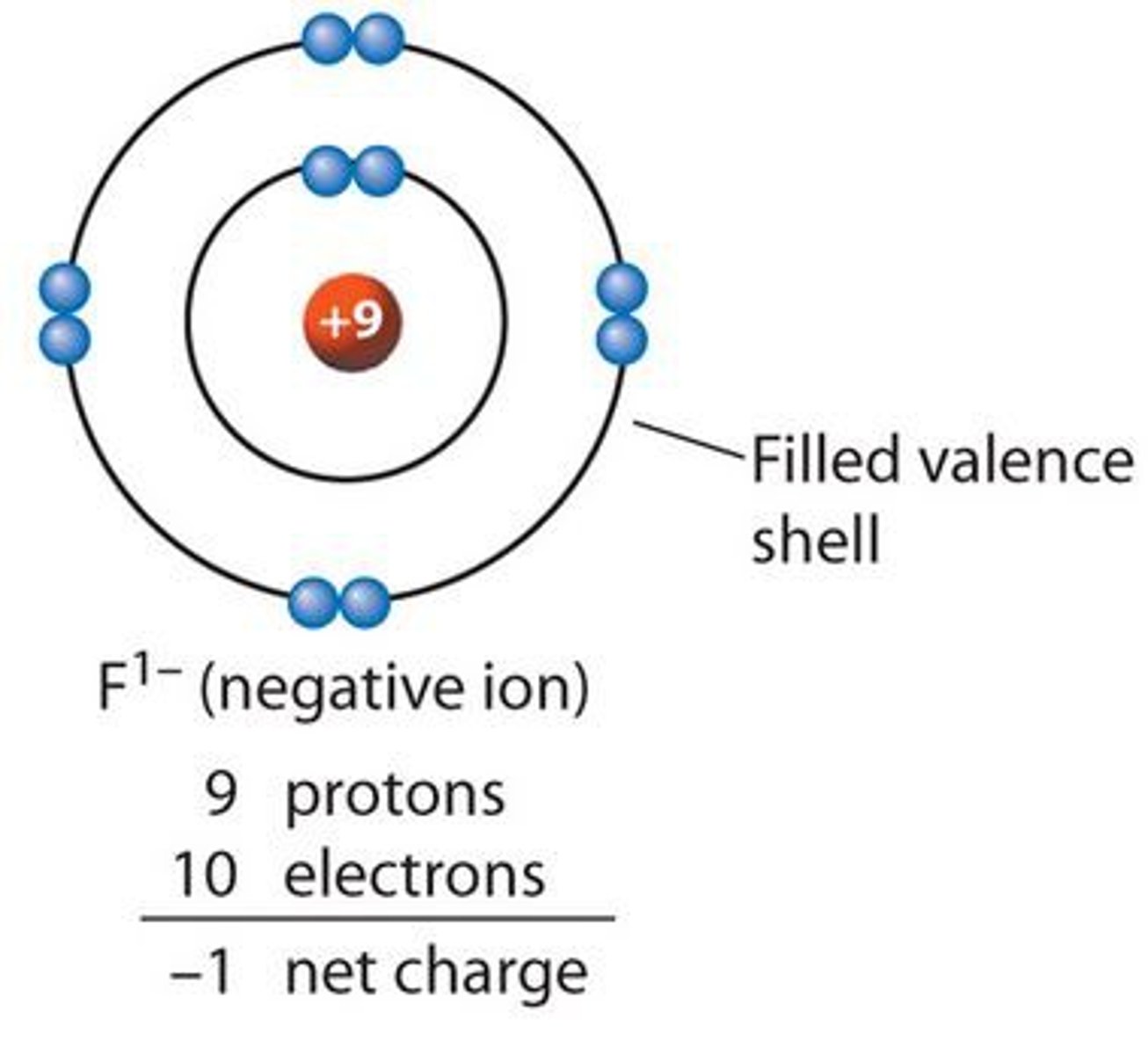
ionic bond
Formed when one or more electrons are transferred from one atom to another
cation
A positively charged ion
anion
A negatively charged ion
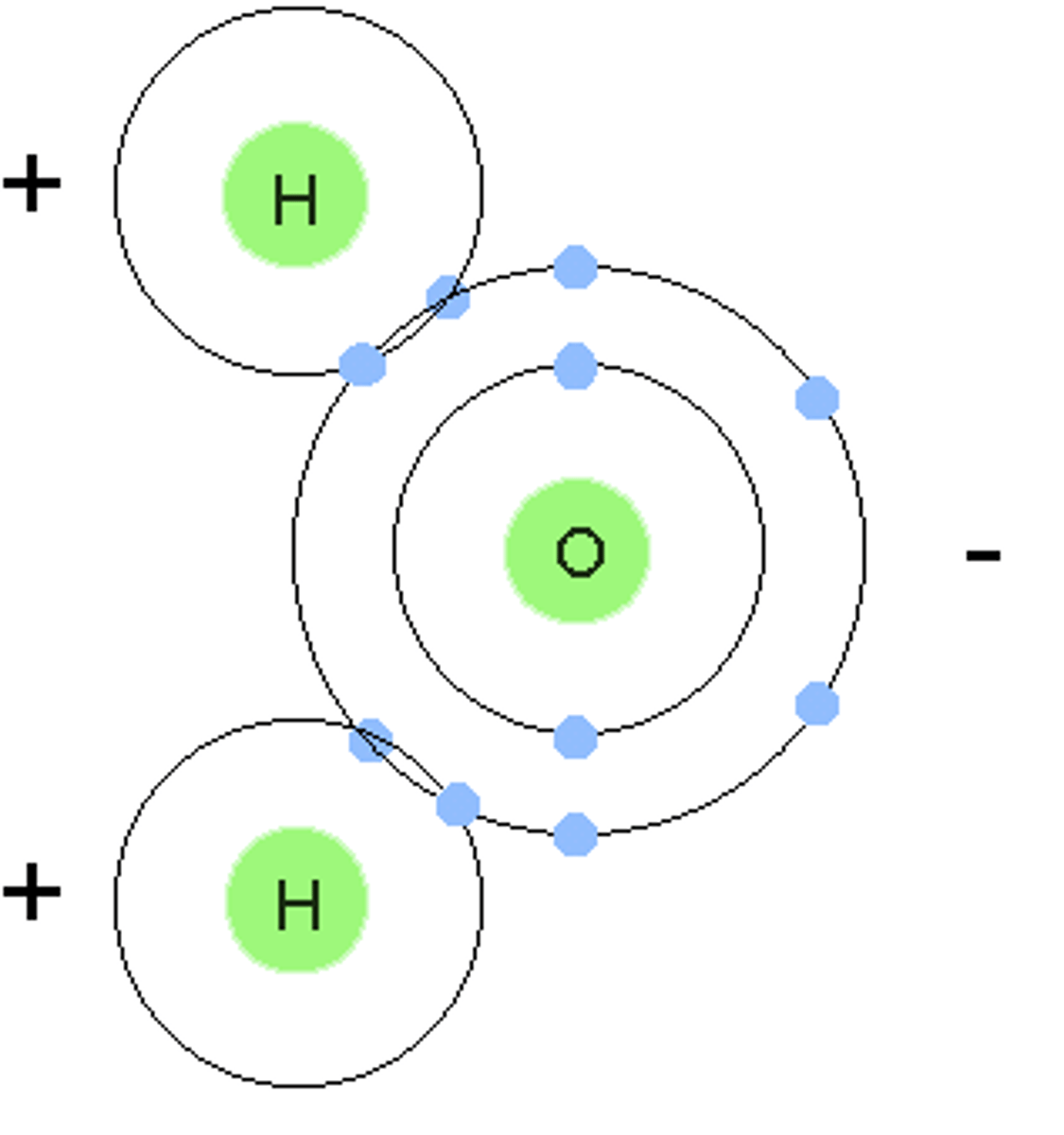
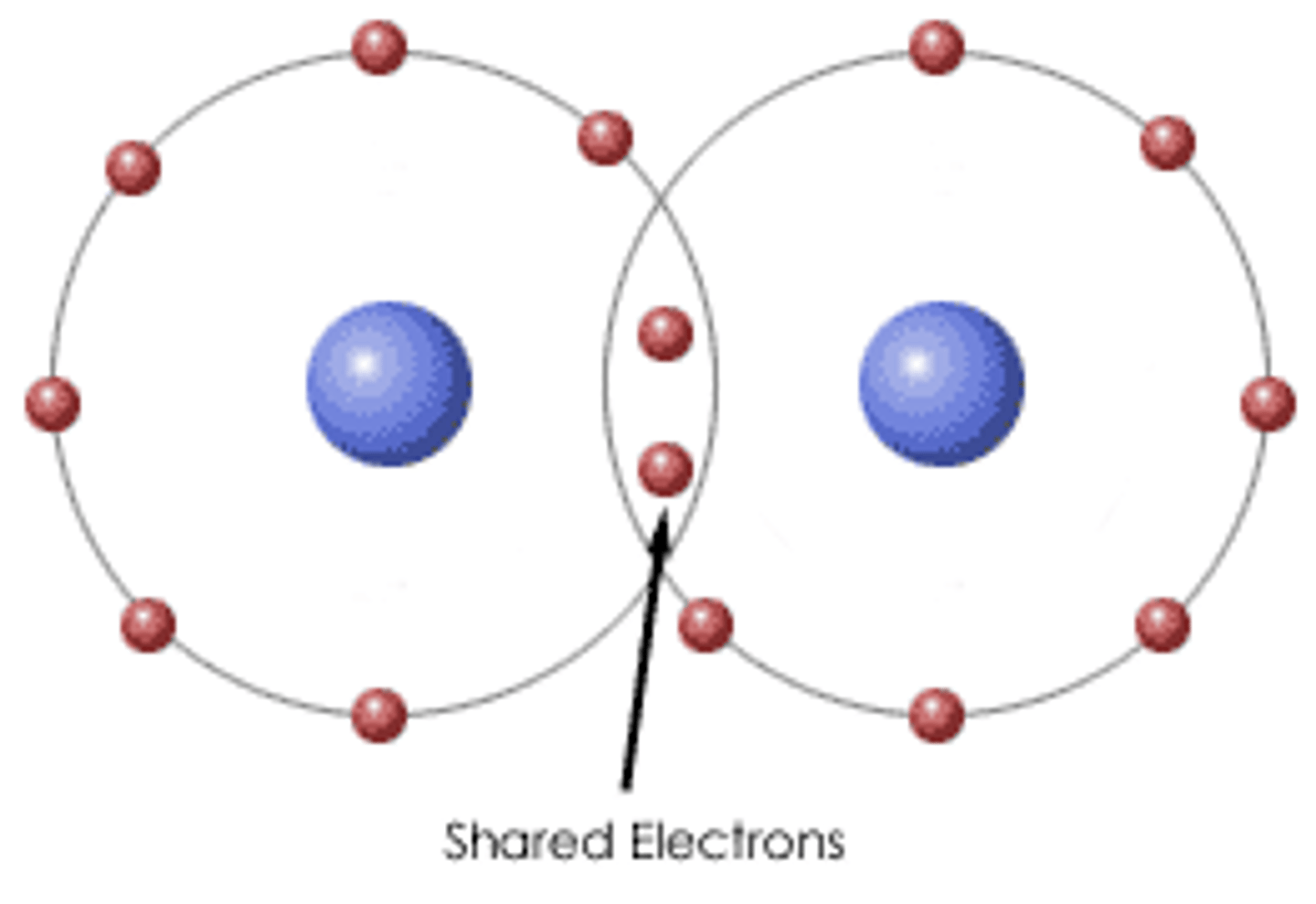
covalent bond
A chemical bond that involves sharing a pair of electrons between atoms in a molecule
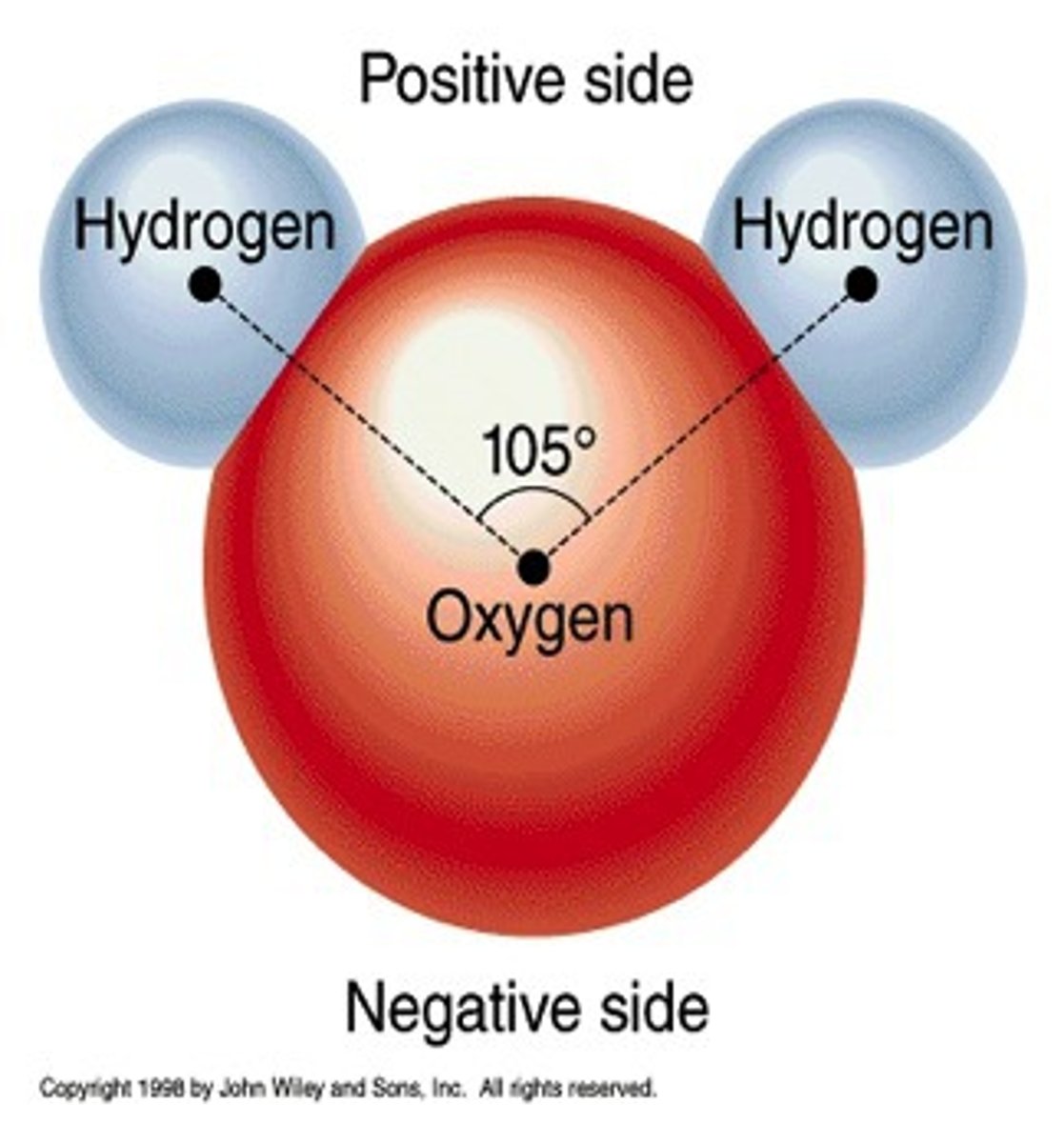
polar covalent bond
A covalent bond in which electrons are not shared equally
nonpolar covalent bond
a covalent bond in which the electrons are shared equally by the two atoms
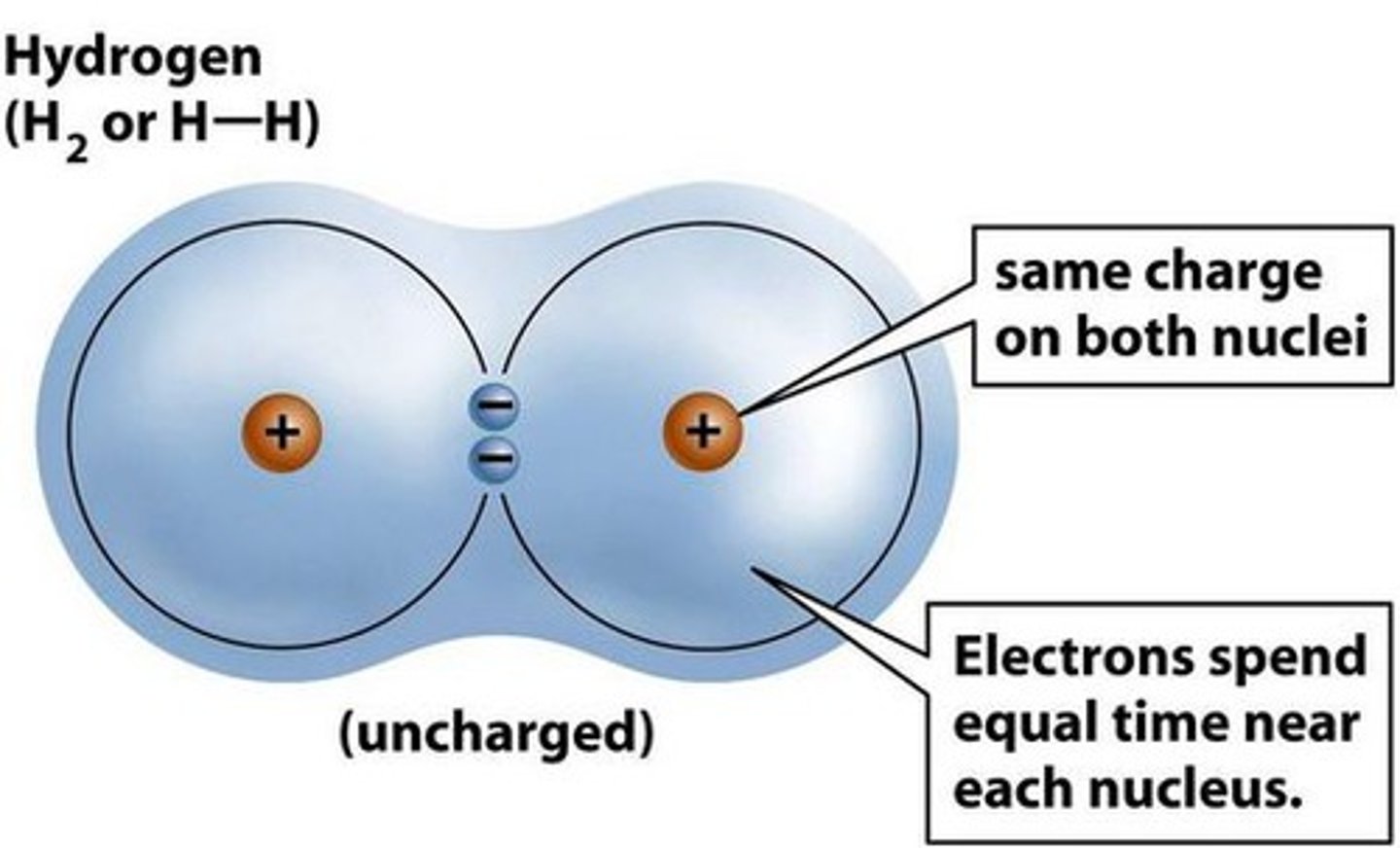
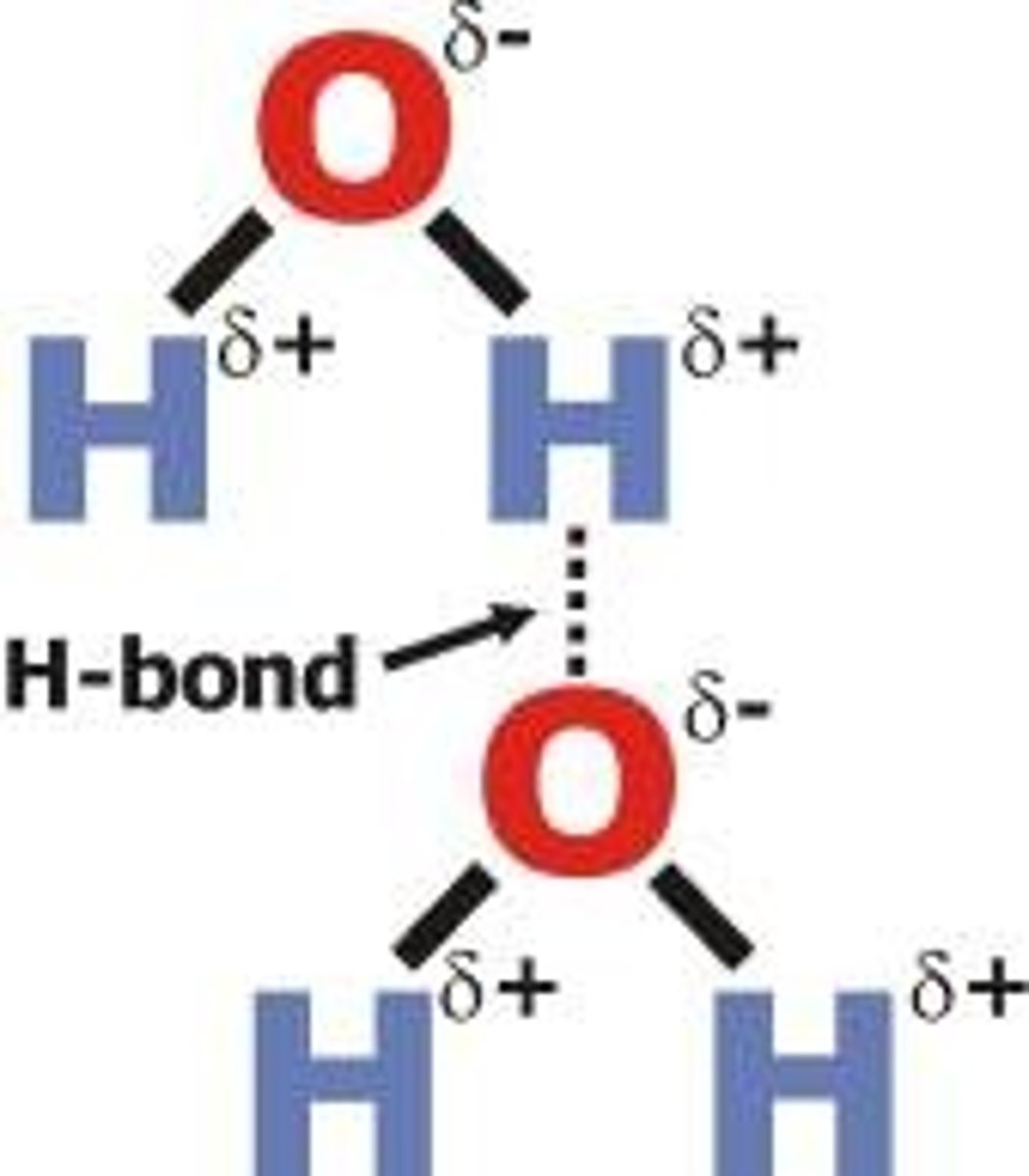
hydrogen bonds
Very weak bonds; occurs when a hydrogen atom in one molecule is attracted to the electrostatic atom in another molecule
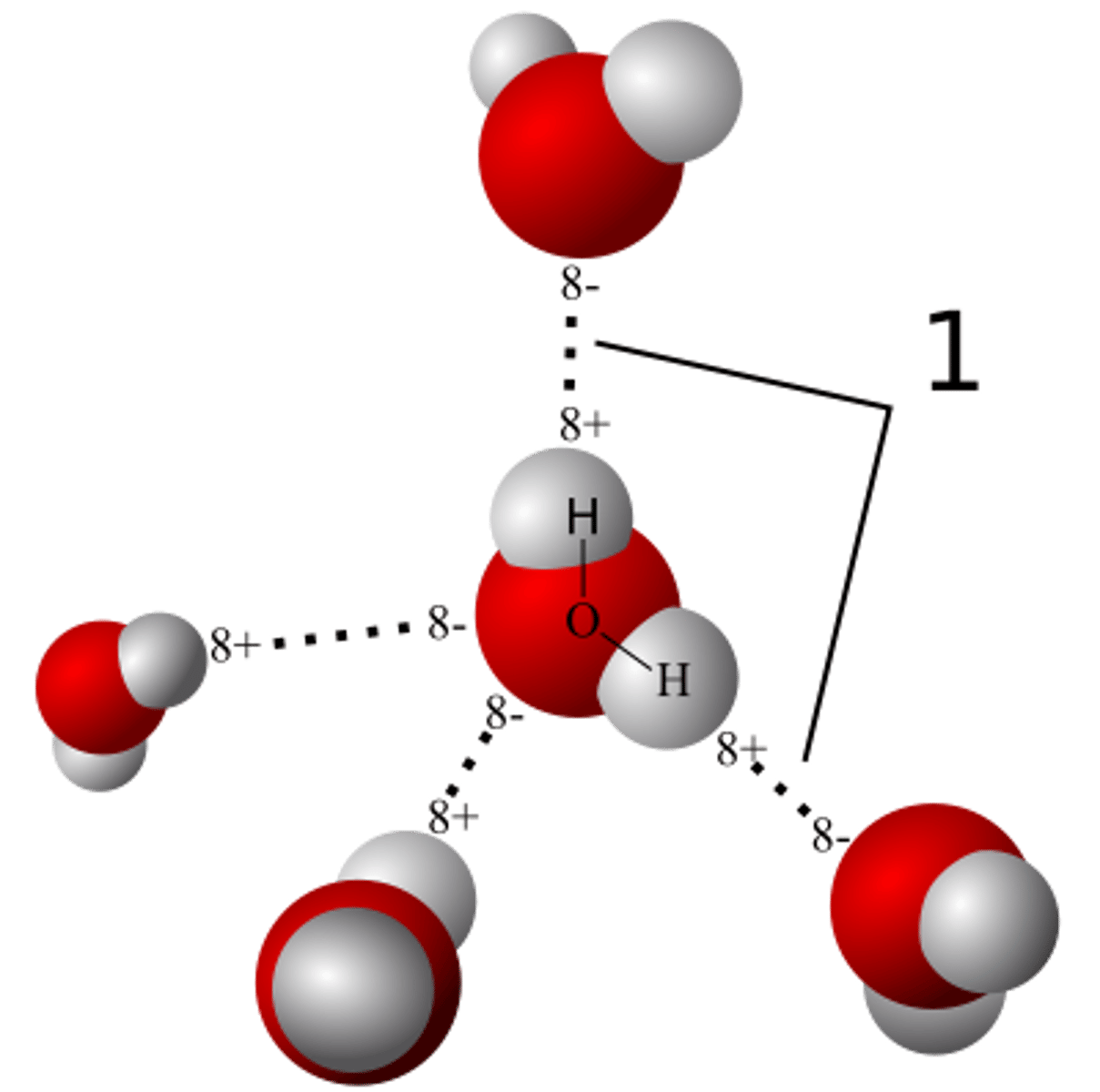
reactant
A chemical substance that is present at the start of a chemical reaction
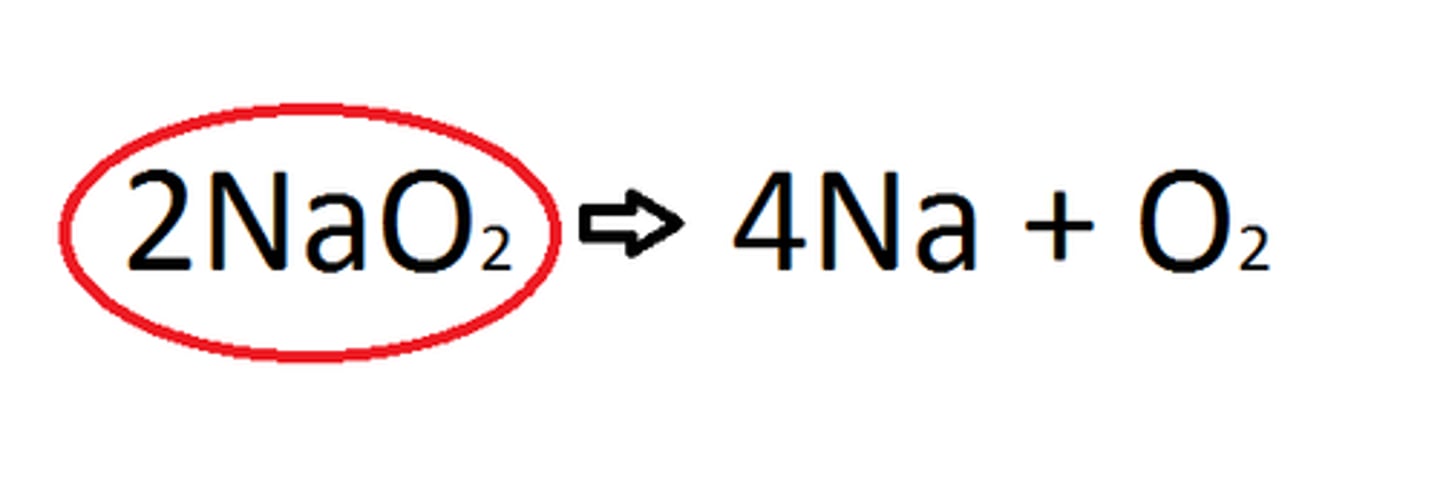
product
A substance produced in a chemical reaction
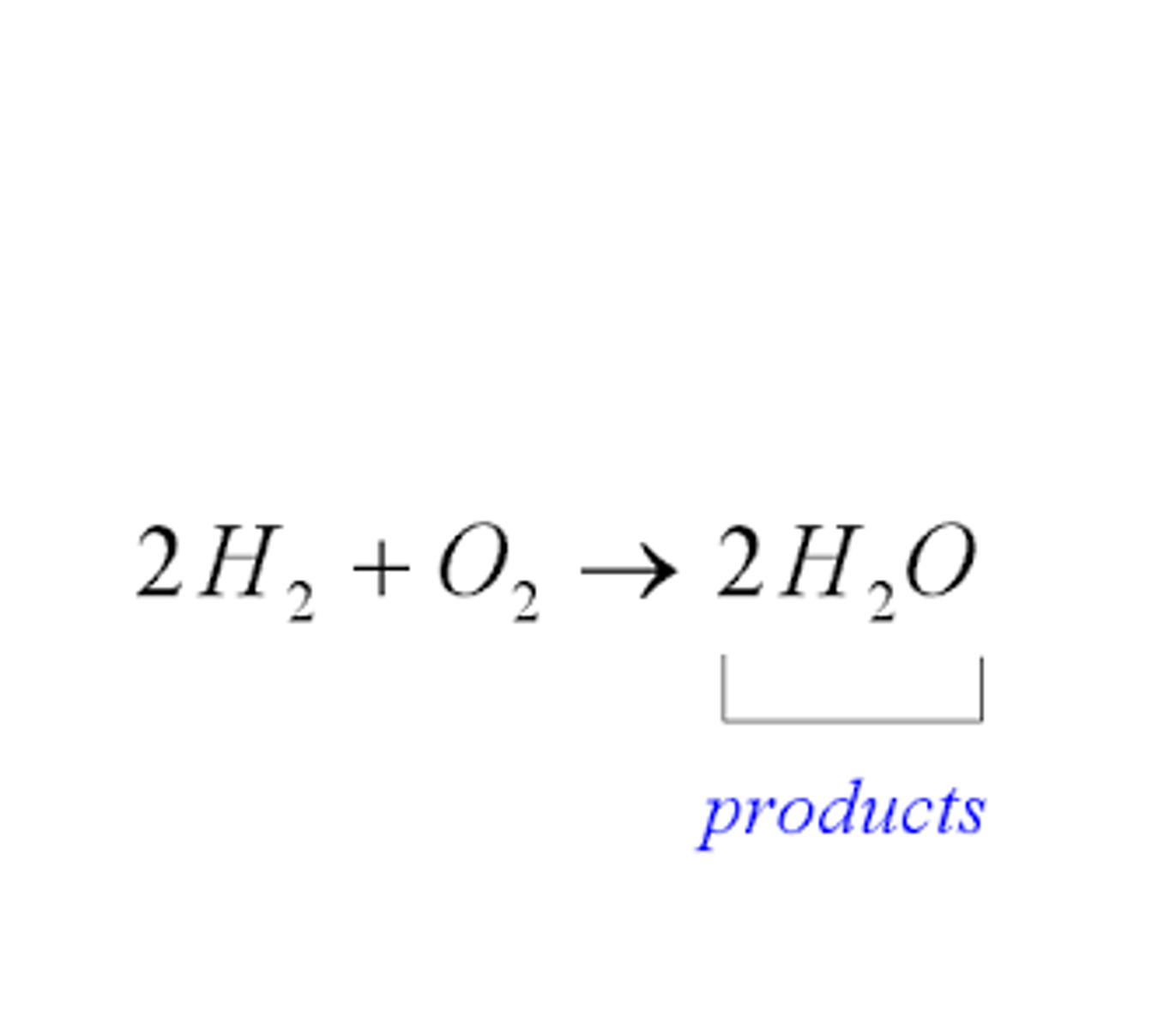
synthesis reaction
a reaction in which two or more substances combine to form a new compound
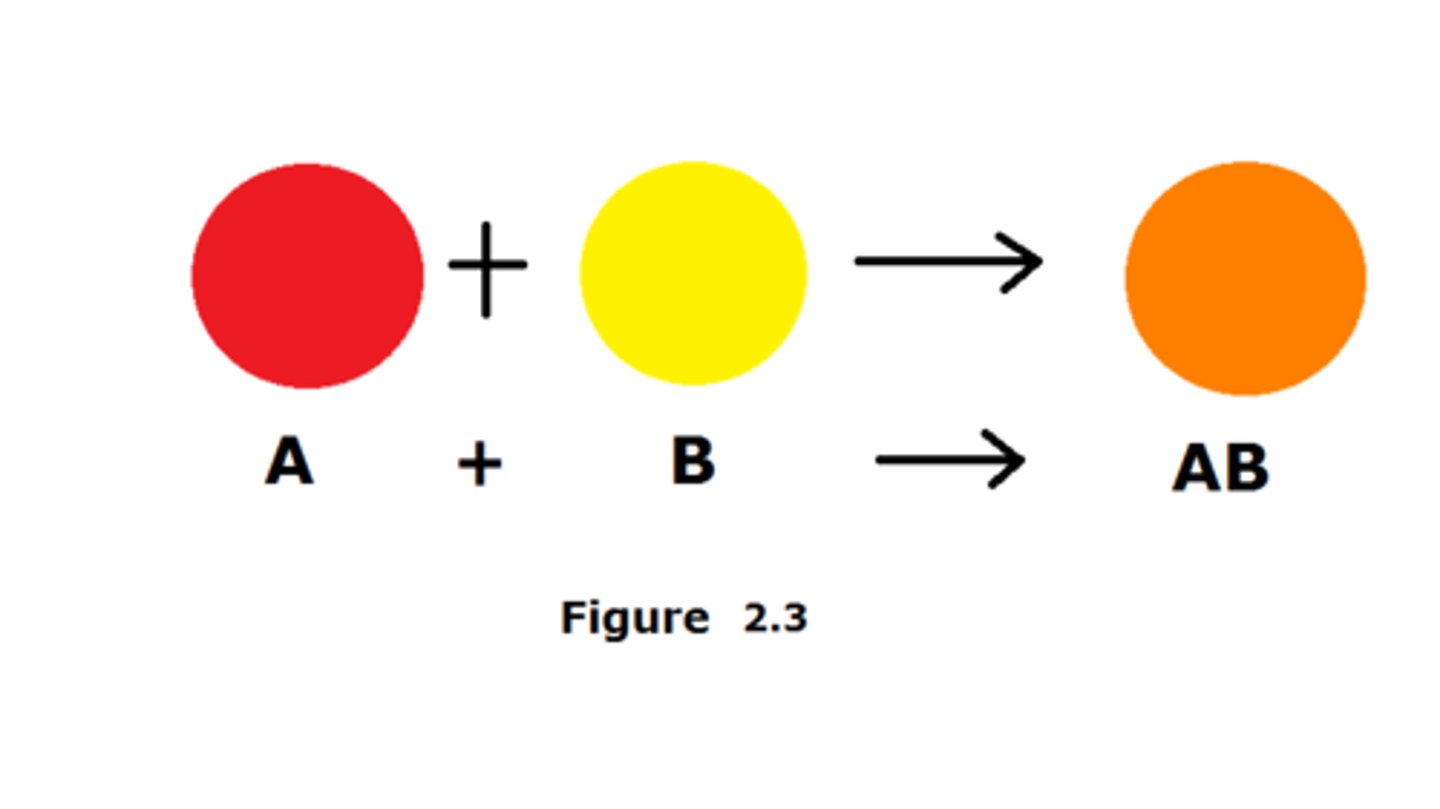
anabolism
Energy requiring phase of metabolism, in which atoms or molecules combine to form a larger, more complex molecule; A + B -> AB (synthesis reaction); always involves bond formation and storing energy
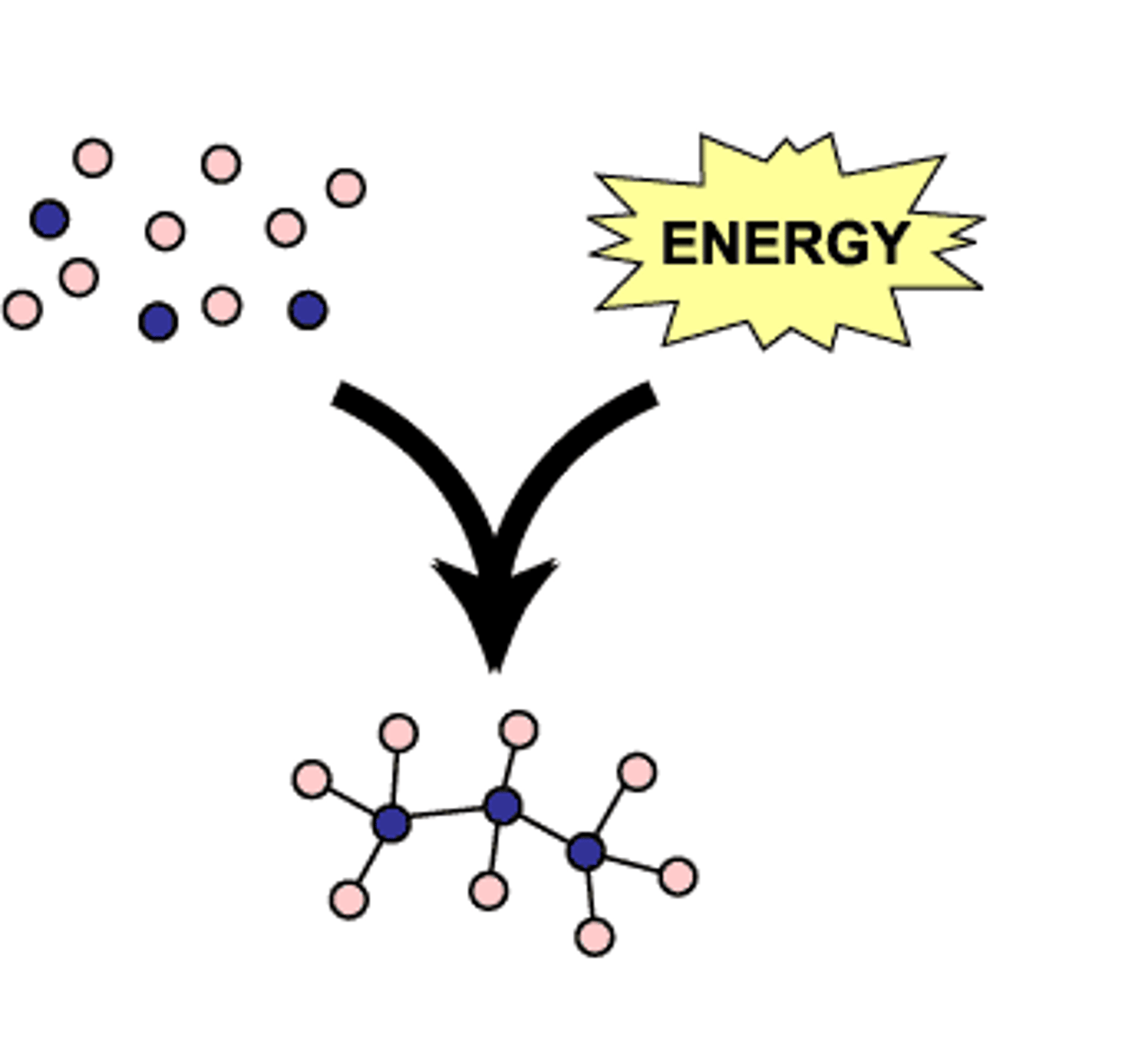
decomposition reaction
a reaction in which a single compound breaks down to form two or more simpler substances
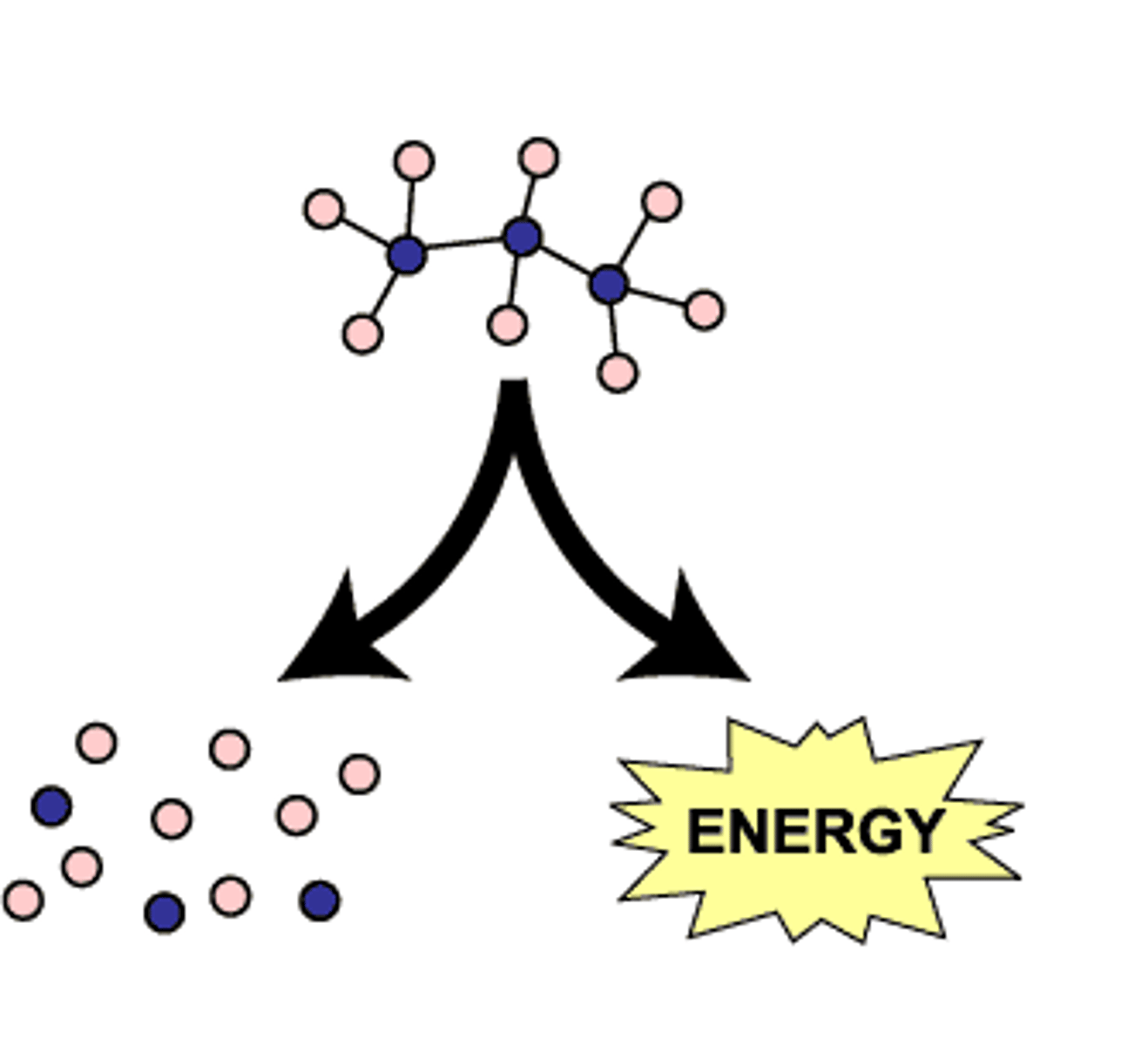
catabolism
Energy releasing phase of metabolism in which a molecule is broken down into smaller molecules; AB -> A + B (reverse of synthesis reactions); always involves breaking of bonds and releasing energy
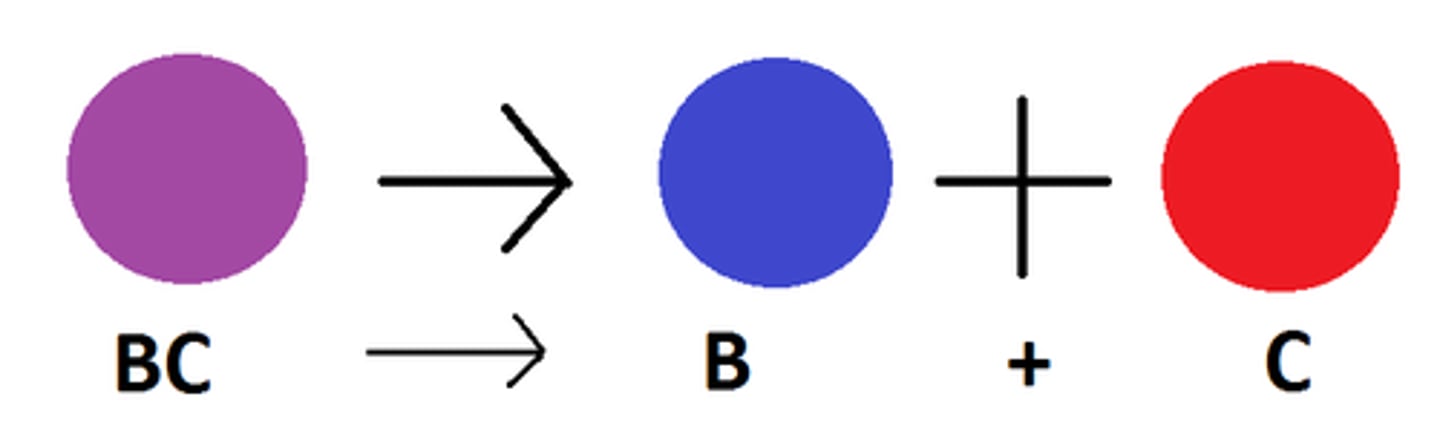
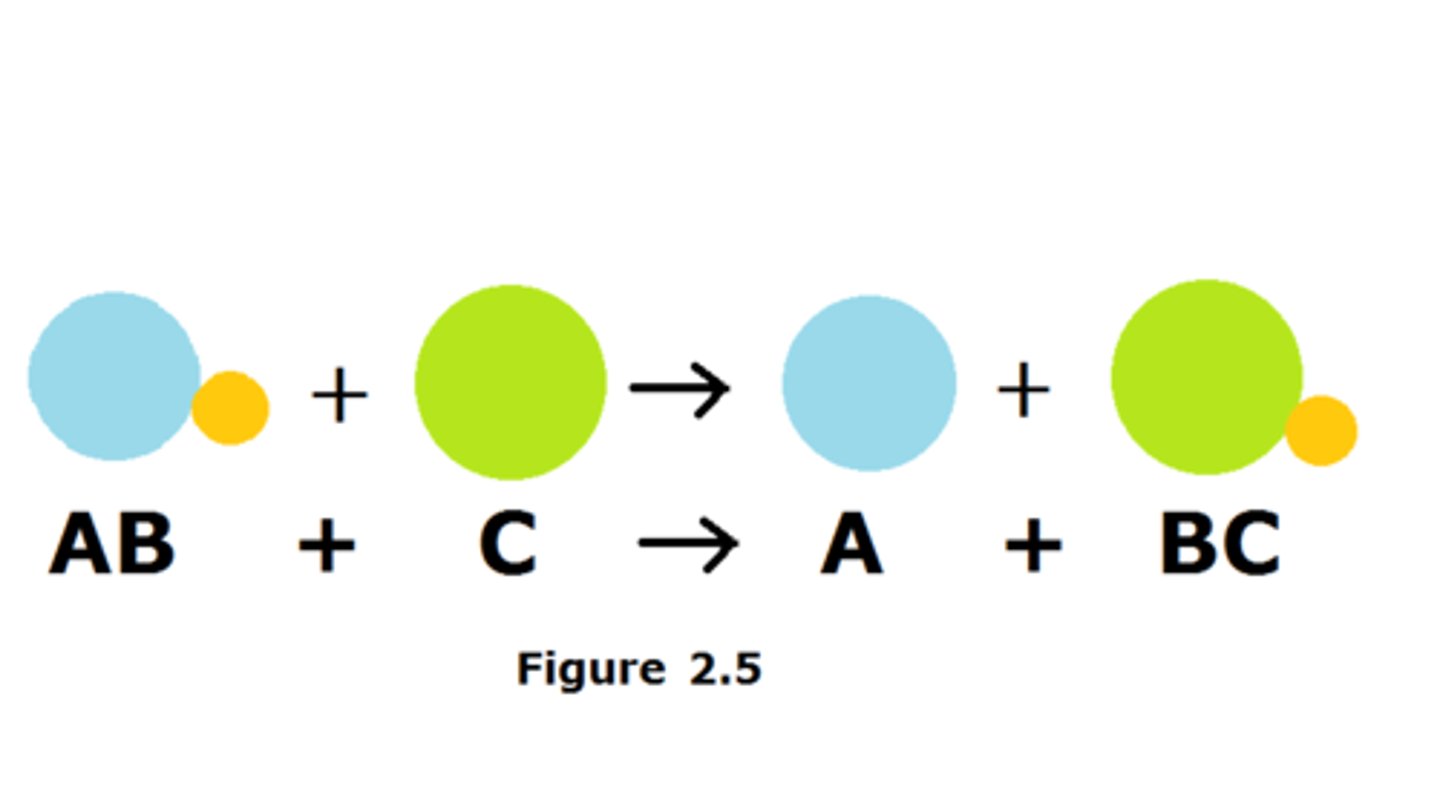
exchange reaction
Parts of the reacting molecules are shuffled around to produce new products
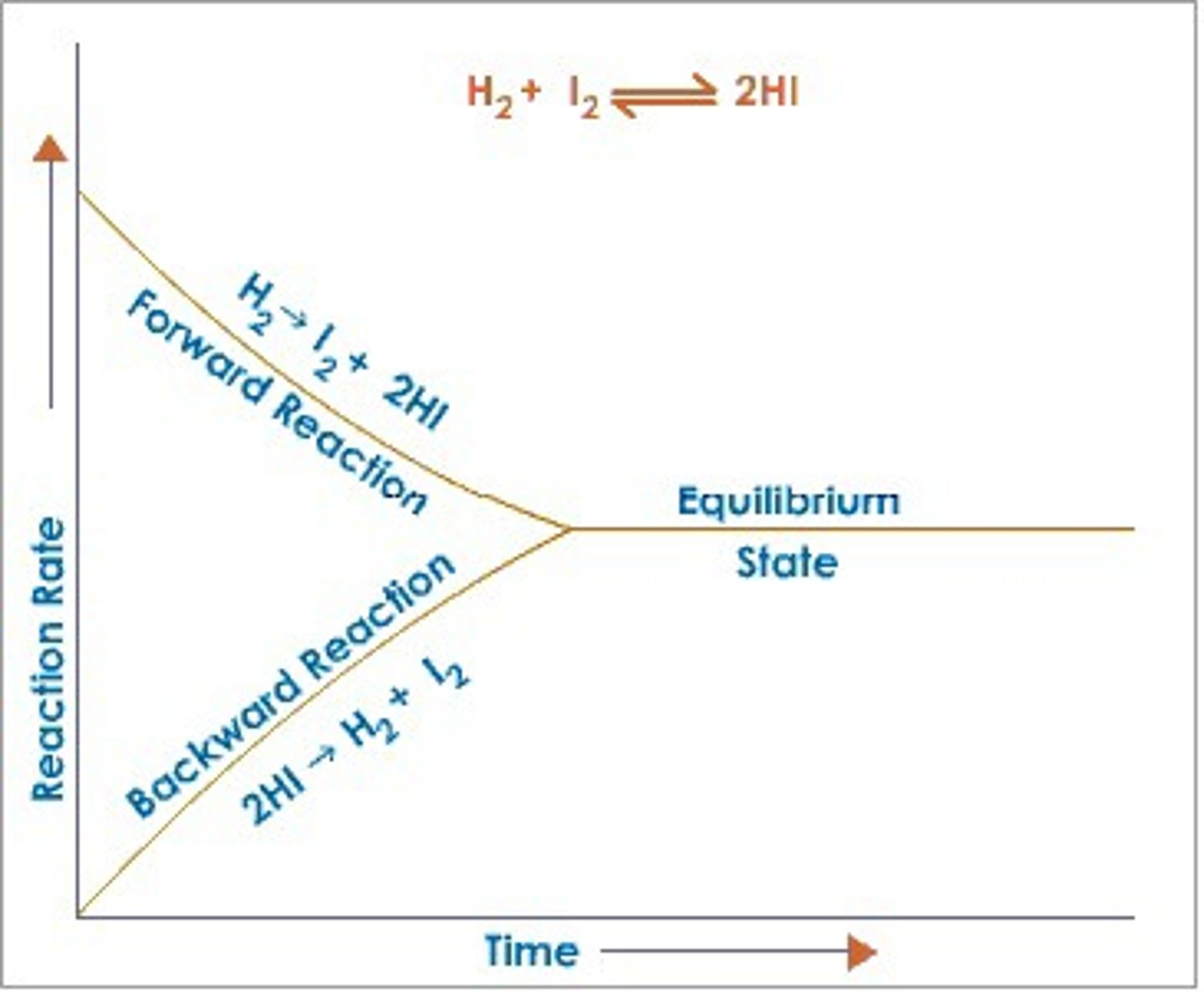
chemical equilibrium
In a chemical reaction, the state in which the rate of the forward reaction equals the rate of the reverse reaction, so that the relative concentrations of the reactants and products do not change with time.
Why are biological reactions sometimes irreversible?
Energy requirements to go backward are too high or products have been removed
The rate of a chemical reaction depends on
temperature, concentration (collision frequency), particle size and properties, and the use of a catalyst
organic
contains carbon; unique to living systems
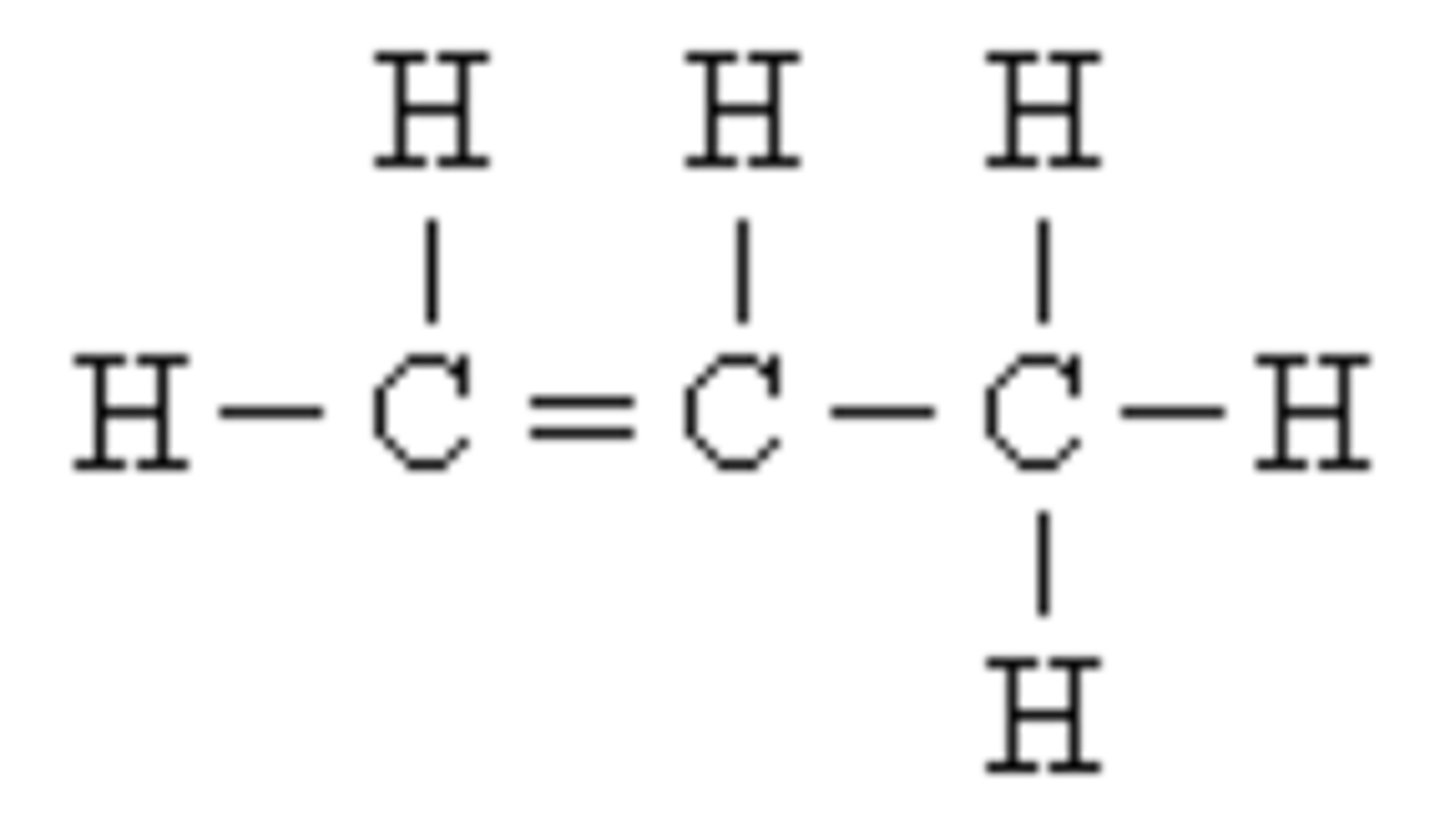
inorganic
Water, salts, bases and acids
water
most abundant and important inorganic compound (60-80% volume of living cells)
properties of water
absorb and release heat slowly, participates in chemical reactions, and cushions body organs.
Hydrophilic
"water-loving"; pertaining to polar or charged molecules (or parts of molecules) that are soluble in water
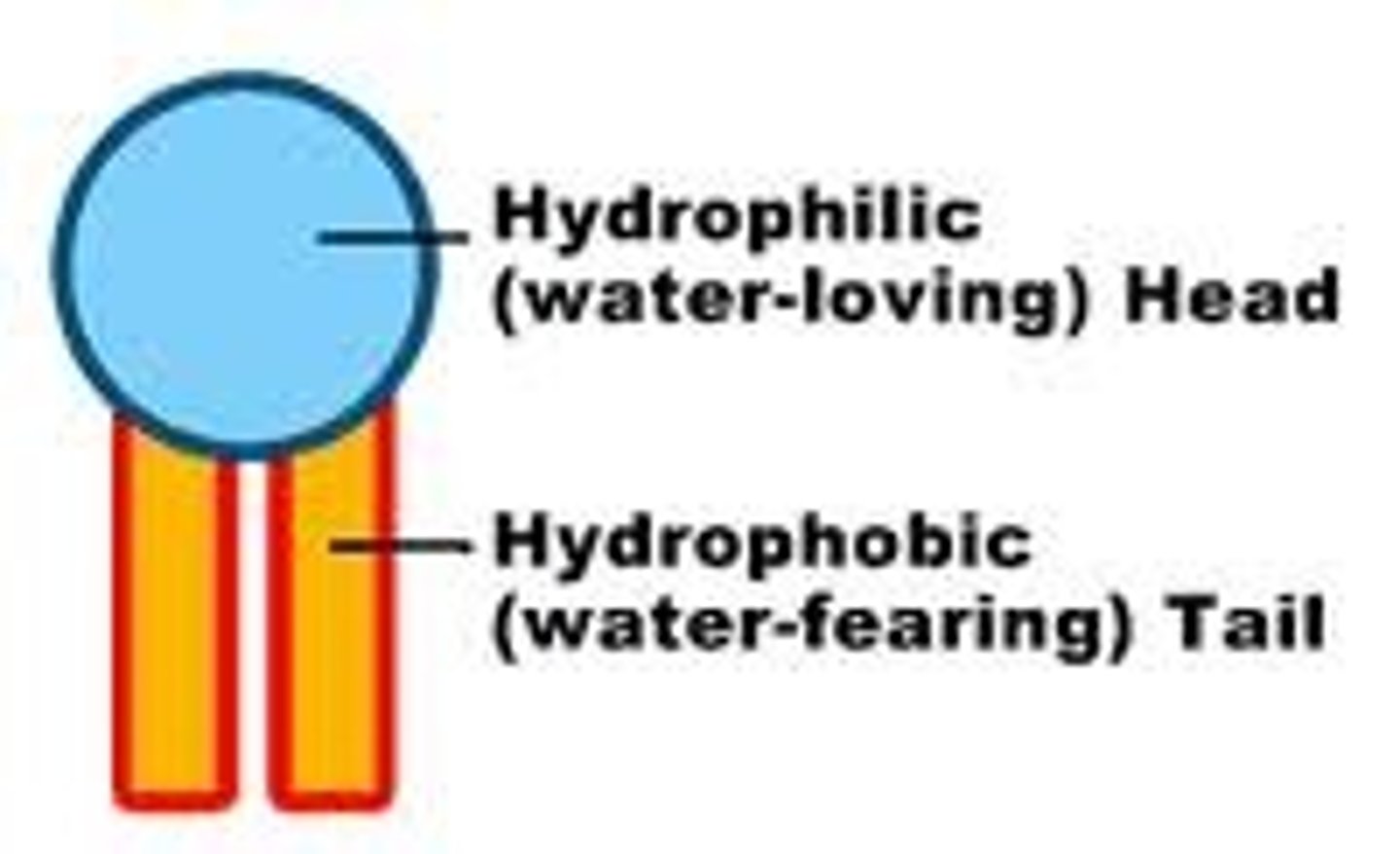
Hydrophobic
water hating; nonpolar substances that will not mix with water.
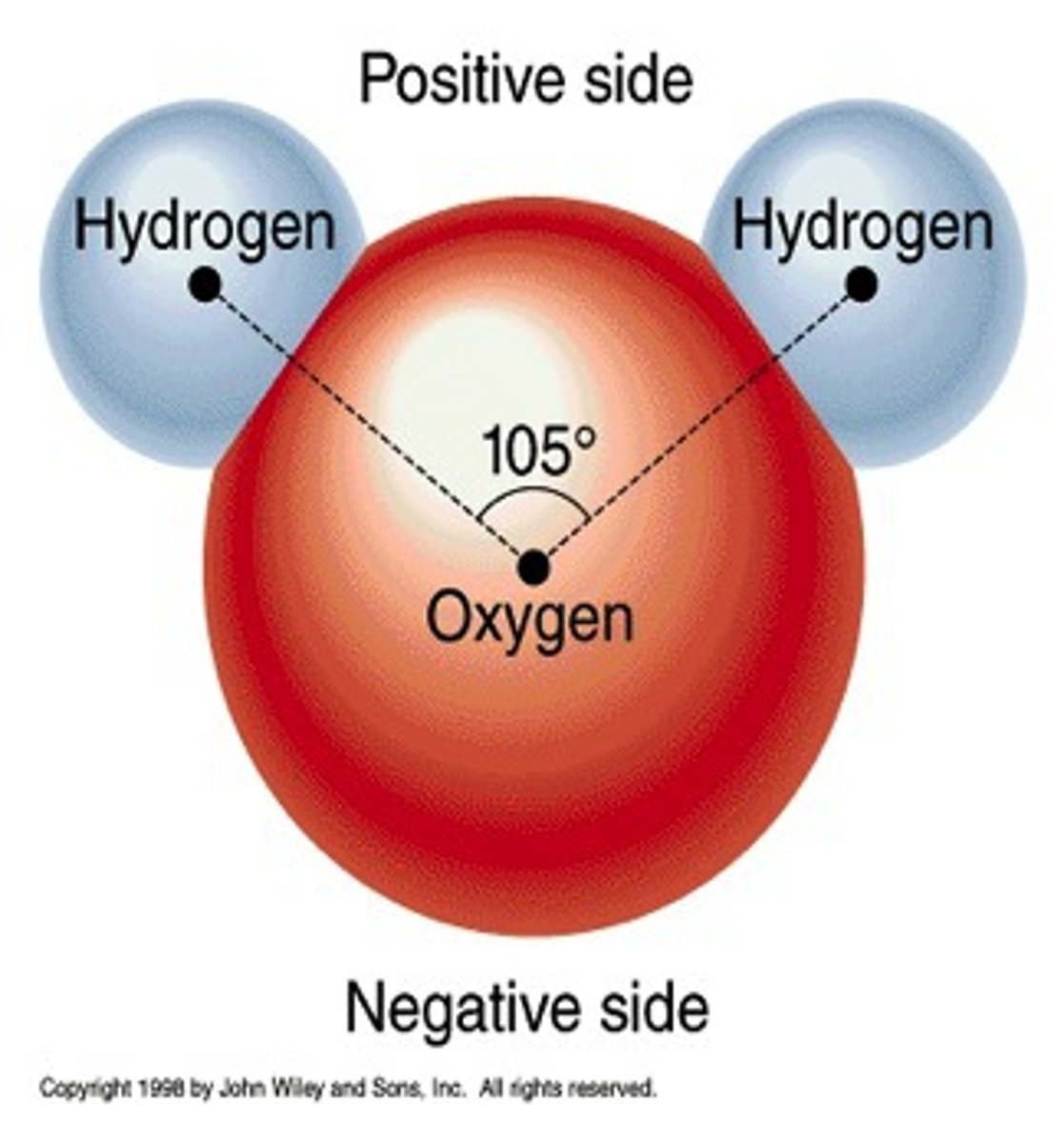
Polar
has an uneven distribution of elctron density
Solvent
the component of a solution that is present in greatest amount; Ex: the ___ for seawater is water and the solute is salt
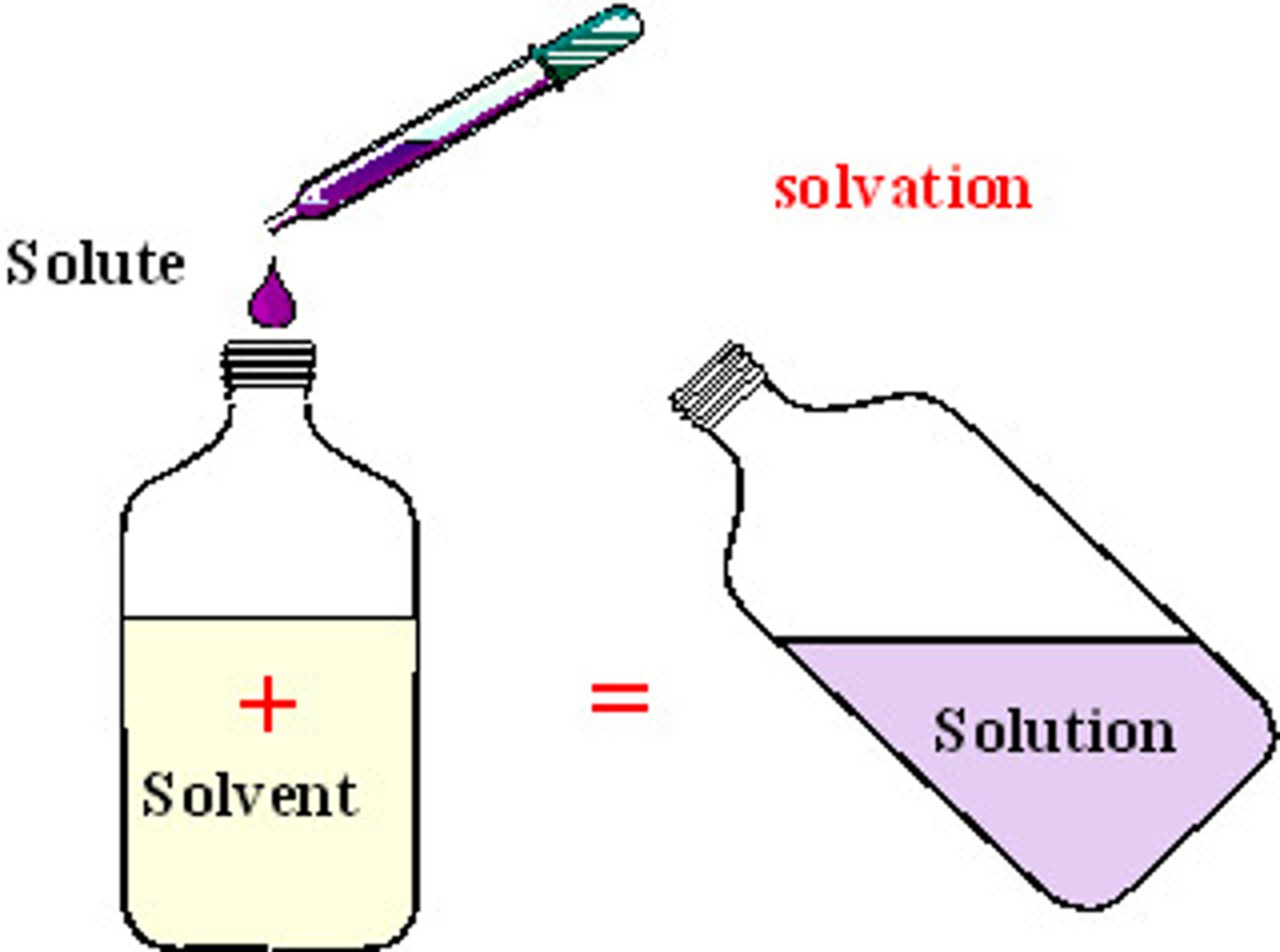
Solute
the minor component in a solution, dissolved in the solvent; Ex: The solvent for lemonade is water and the _________(s) are sugar and lemon juice
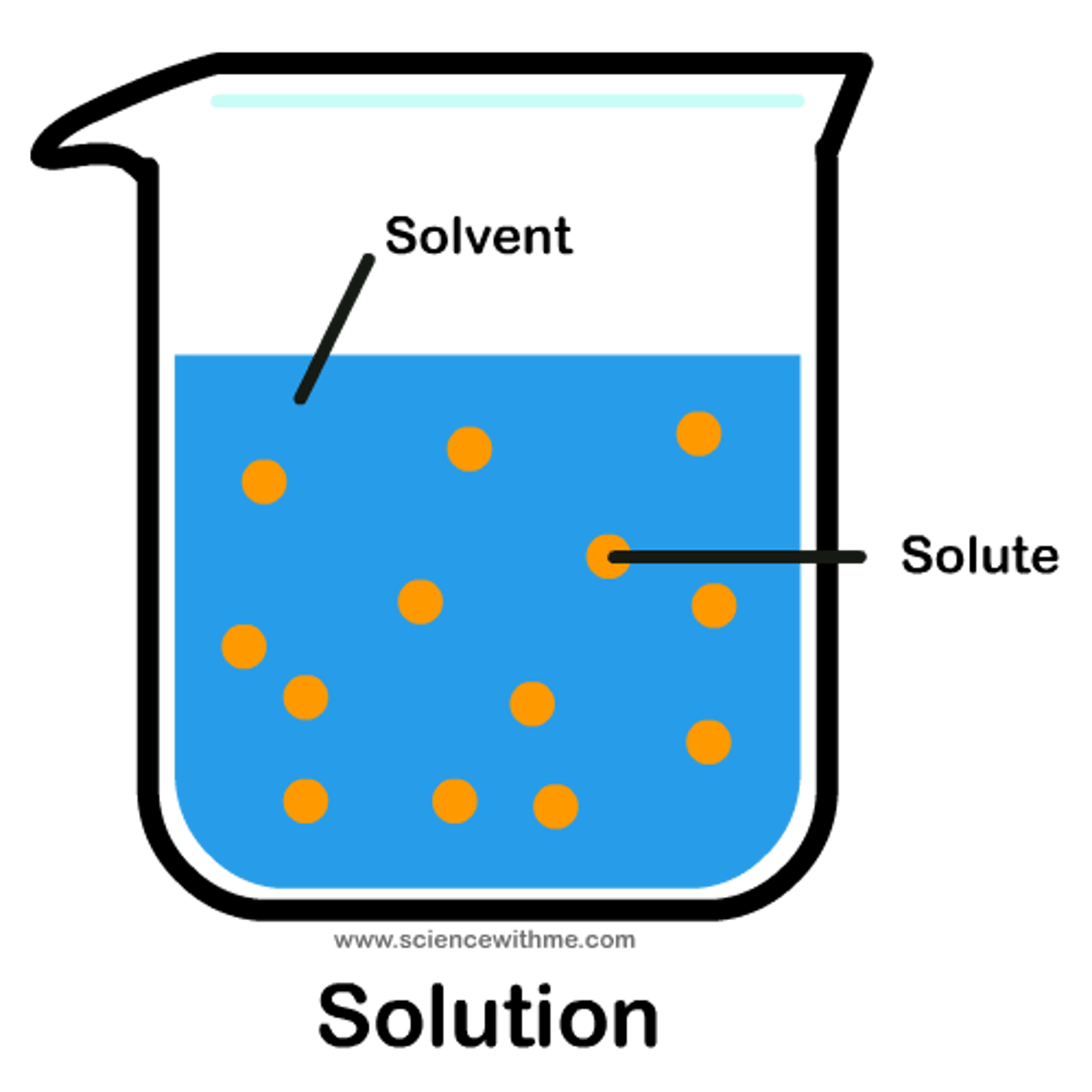
Solution
homogeneous mixture composed of a solute and a solvent; Ex: the solvent for air is nitrogen (78.09% ) and the solutes include oxygen (20.95%), water (1%), and carbon dioxide (0.039%)
Salt
ionic compounds that dissolve in water
Common identifiers: NaCl, CaCo3, KCl

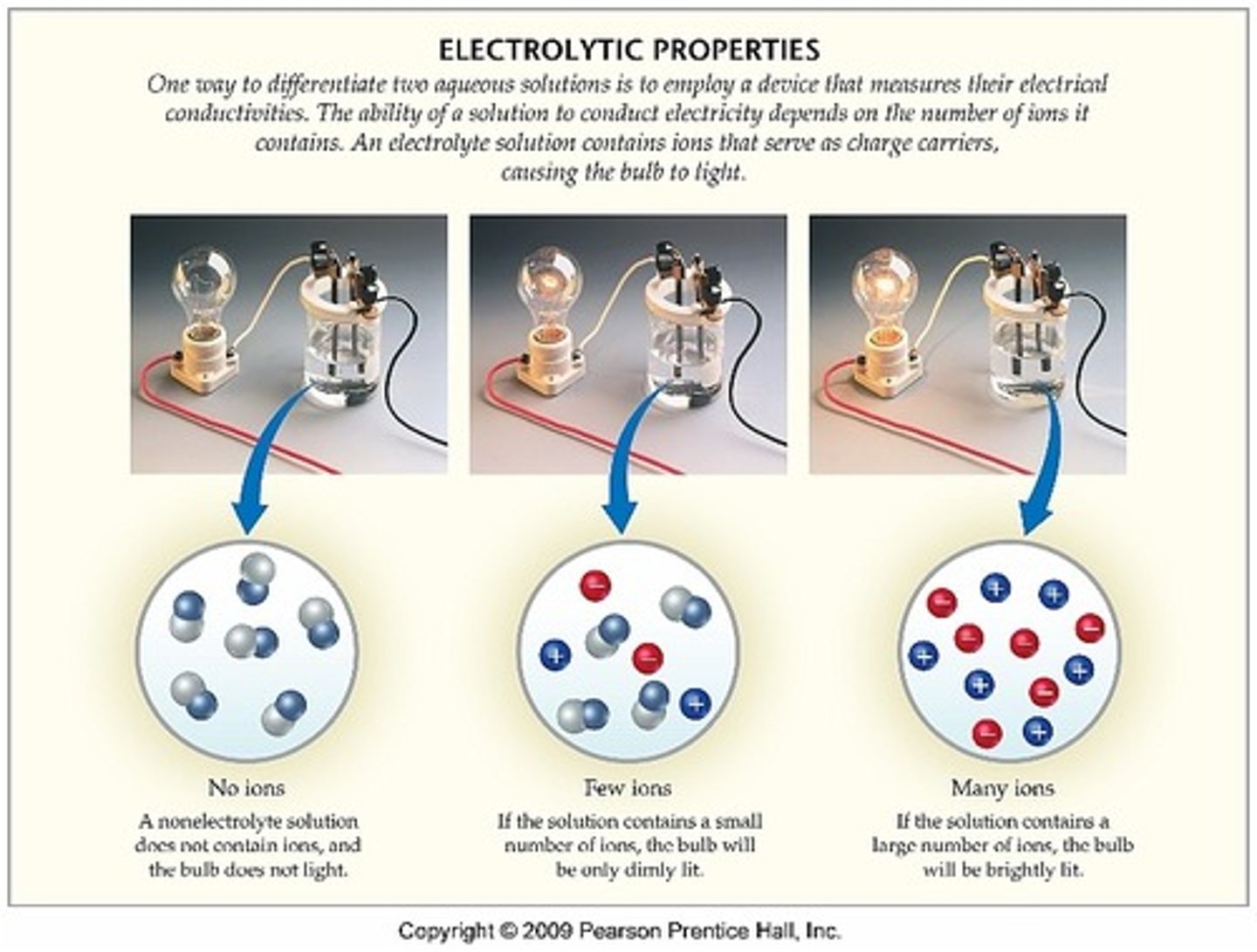
Electrolyte
ions that conduct ELECTRICAL currents in a solution
Acids and Bases
-Both are electrolytes
-Ionize and dissociate in water
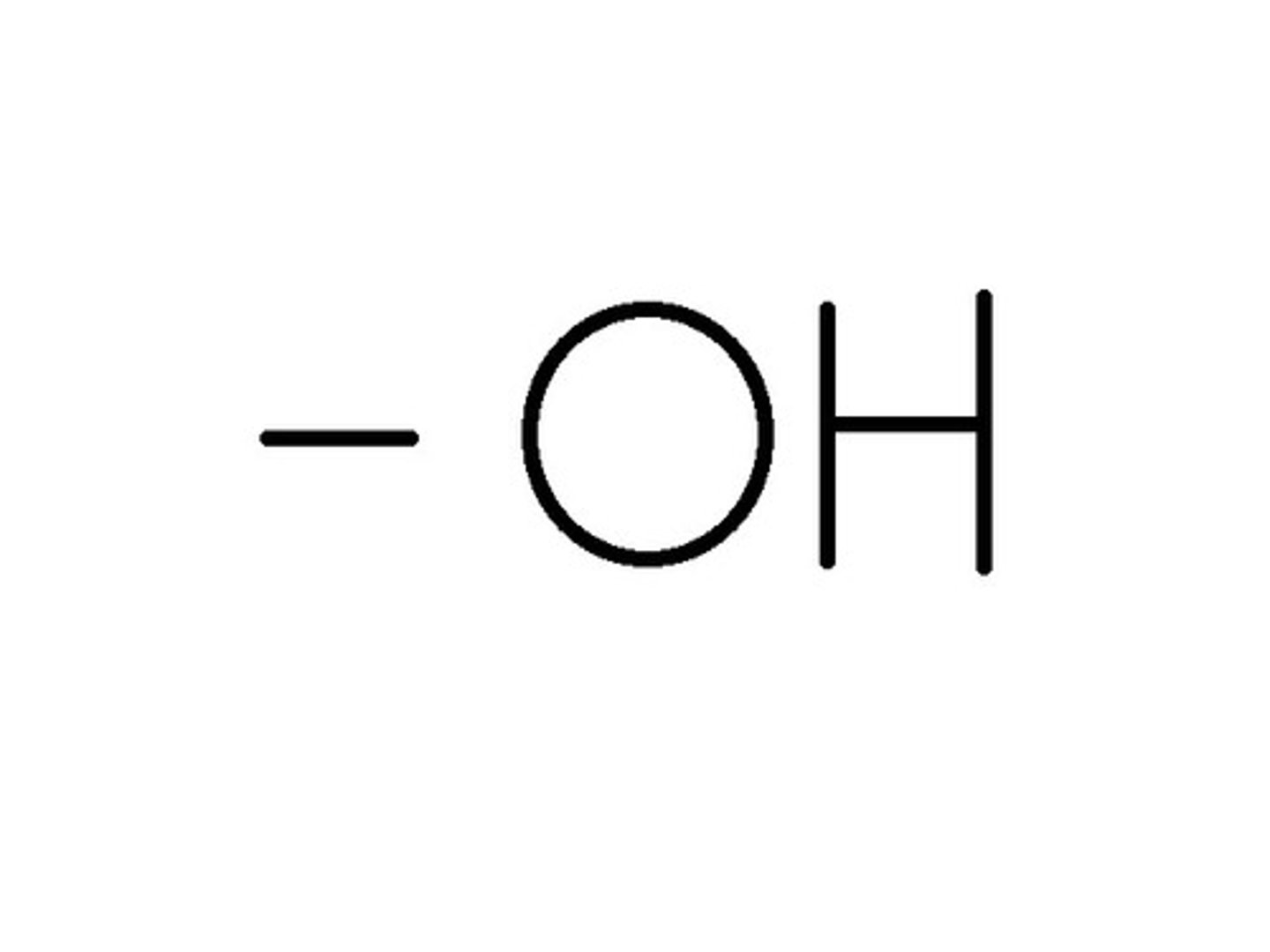
Acids
Proton donors- release H+ in a solution
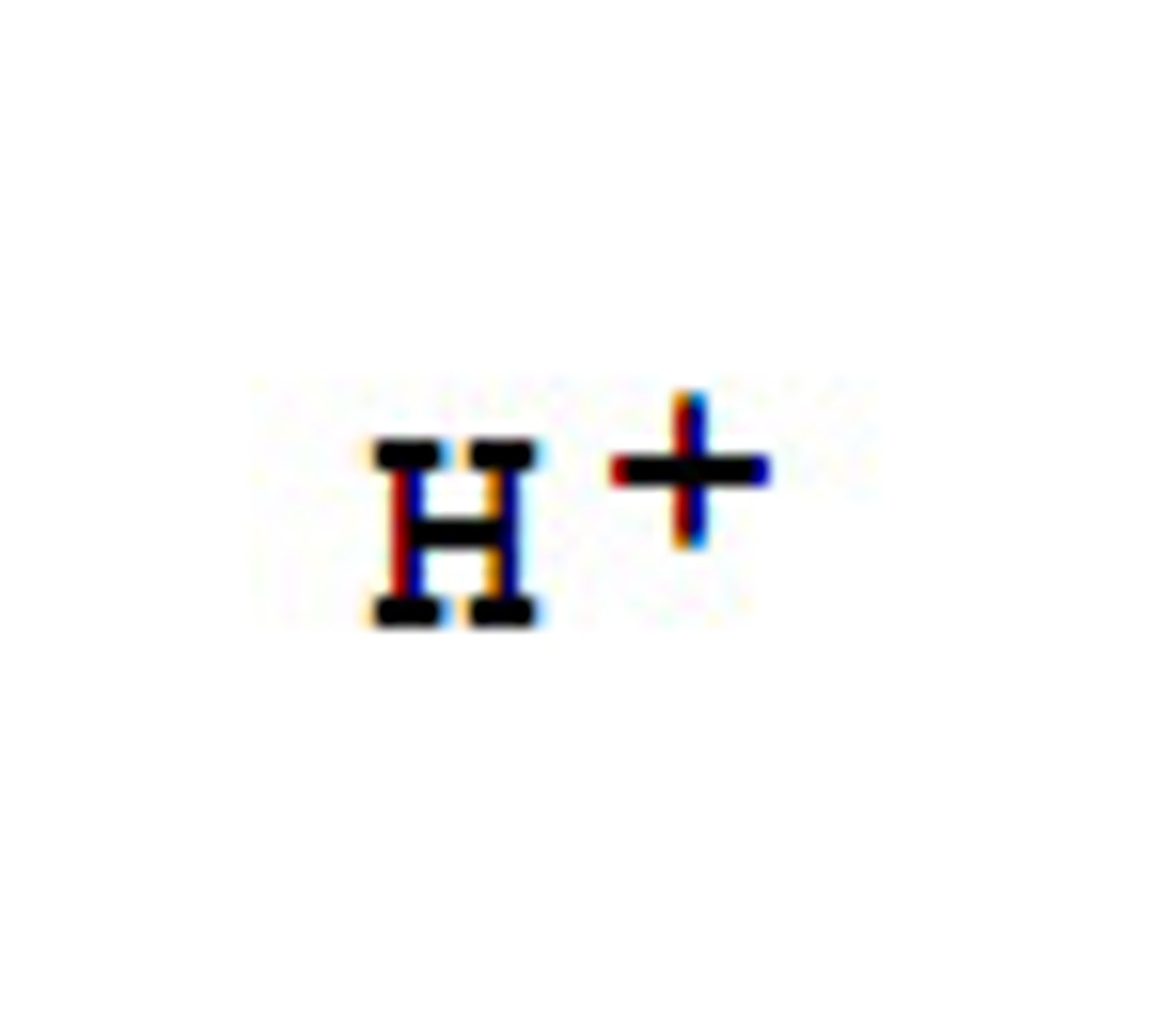
Bases
Proton acceptors (OH-) that take up a H+ from a solution
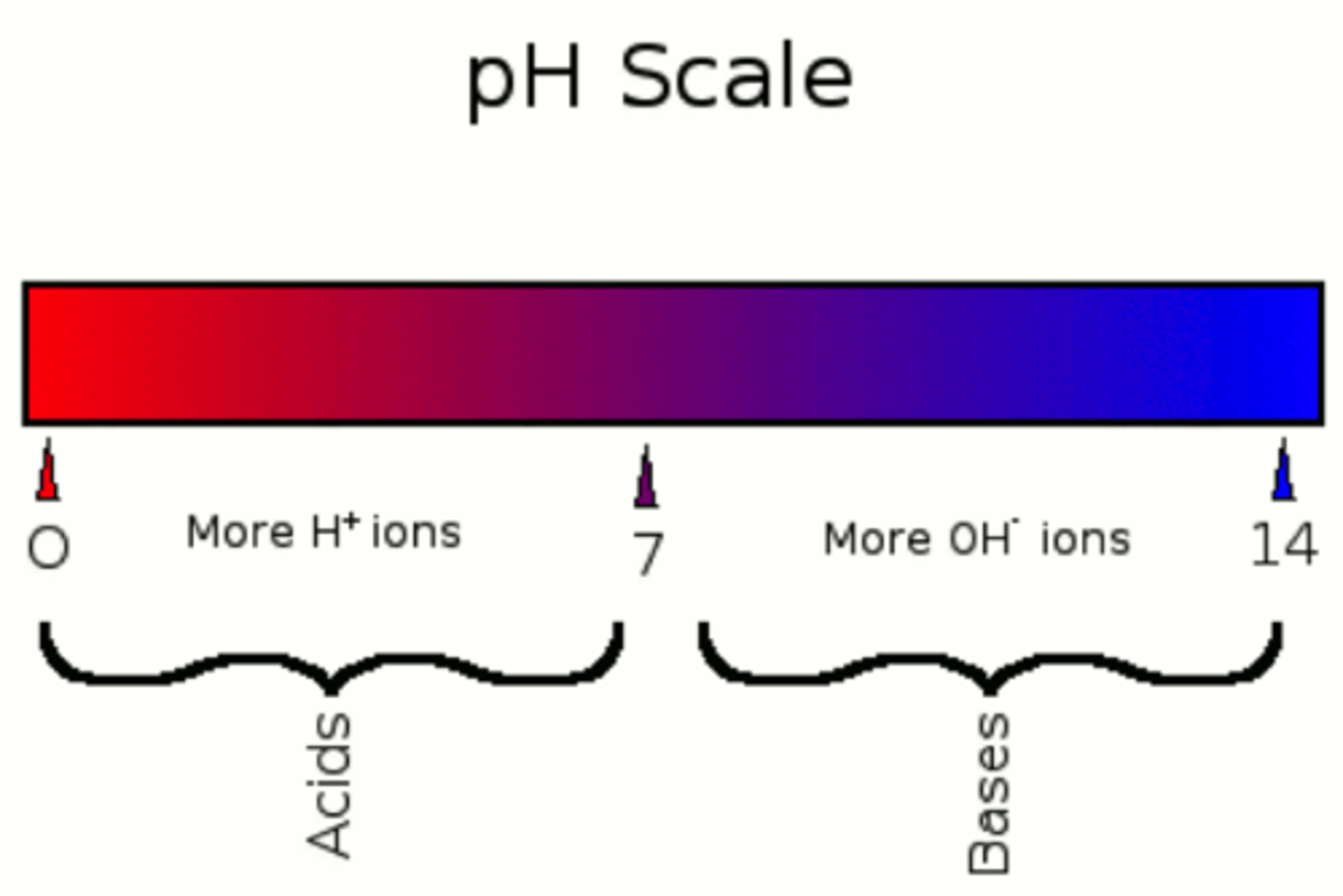
how are acids and bases represented on the pH scale?
As free (H+) increases, then pH decreases (acidity)
As free (H+) decreases, then pH increases (alkalinity, base)
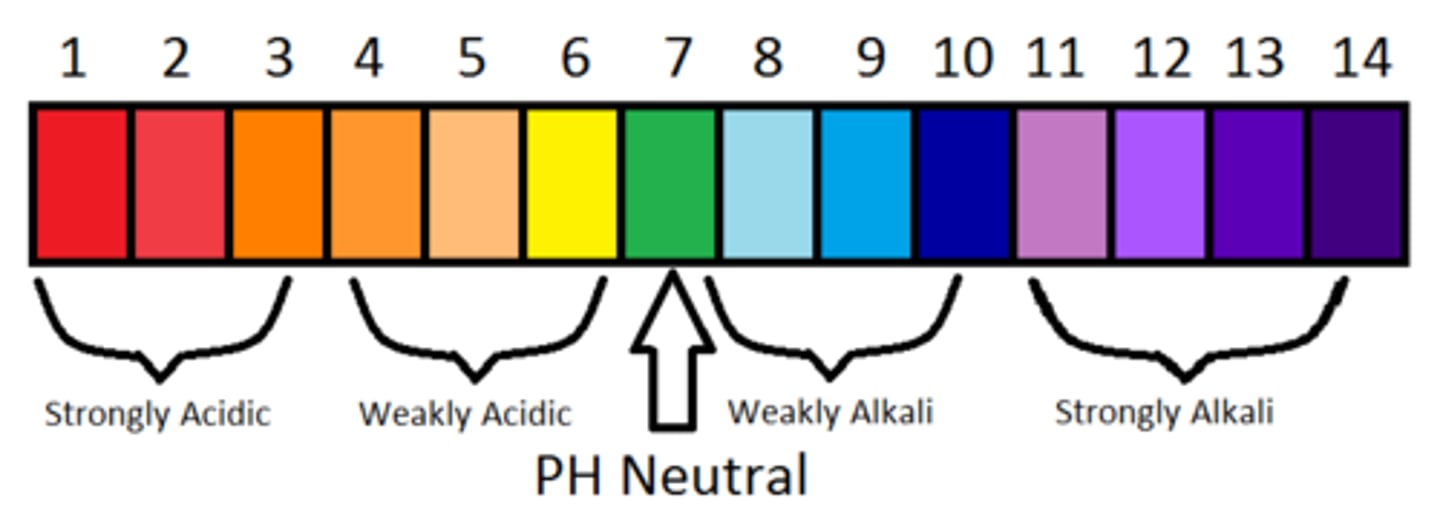
Normal pH of human blood
7.35-7.45 (slightly alkaline)
why is pH homeostasis important?
pH influences every physiological activity in the body;
pH change interferes with cell function and may damage living tissue; pH affects speed of all biological and electrical reactions; even slight change in pH can be fatal
buffer
molecules that resist abrupt and large swings in pH
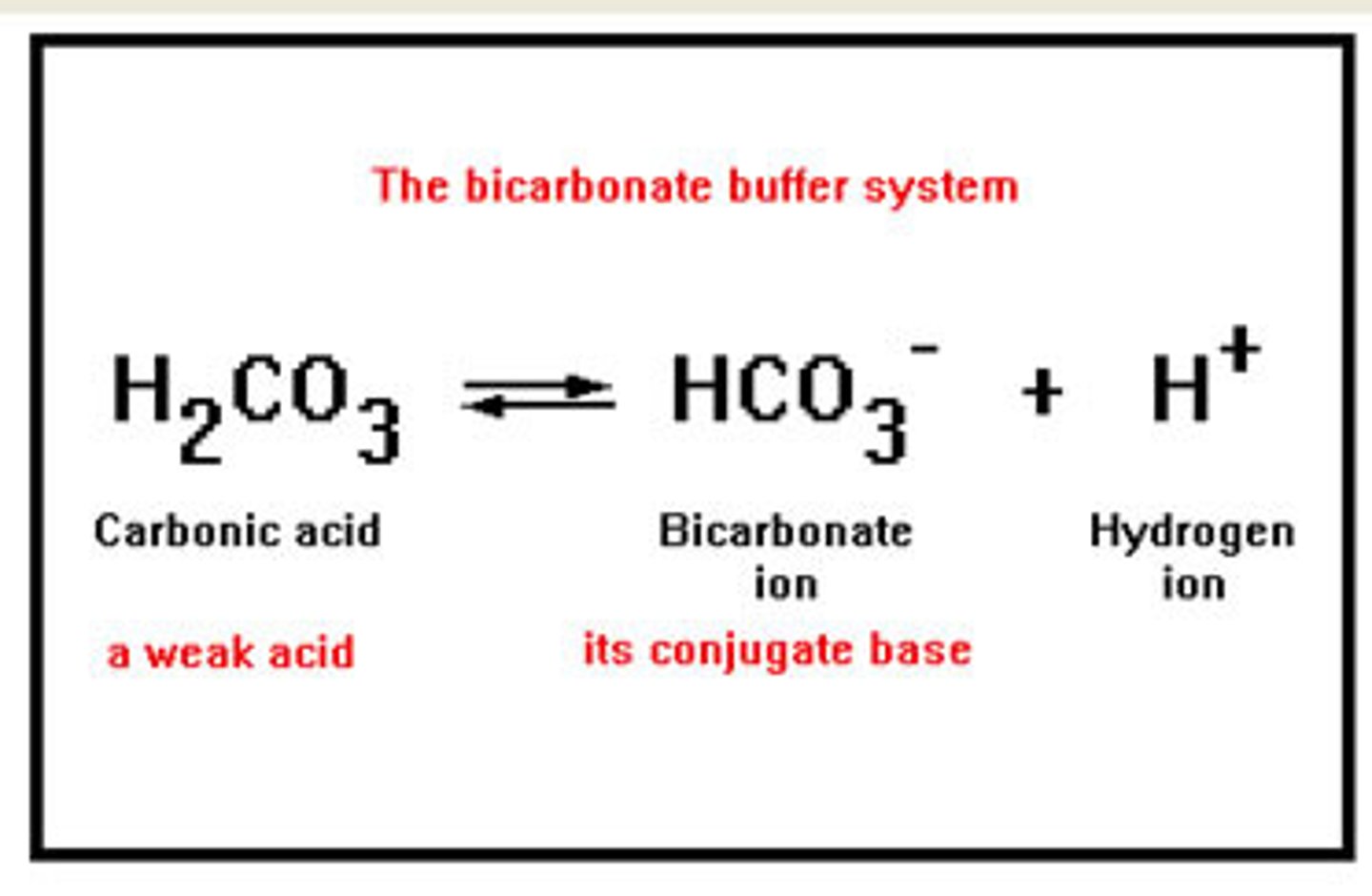
how does a buffer prevent abrupt changes in pH?
by converting strong acids into weak acids and strong bases into weak bases
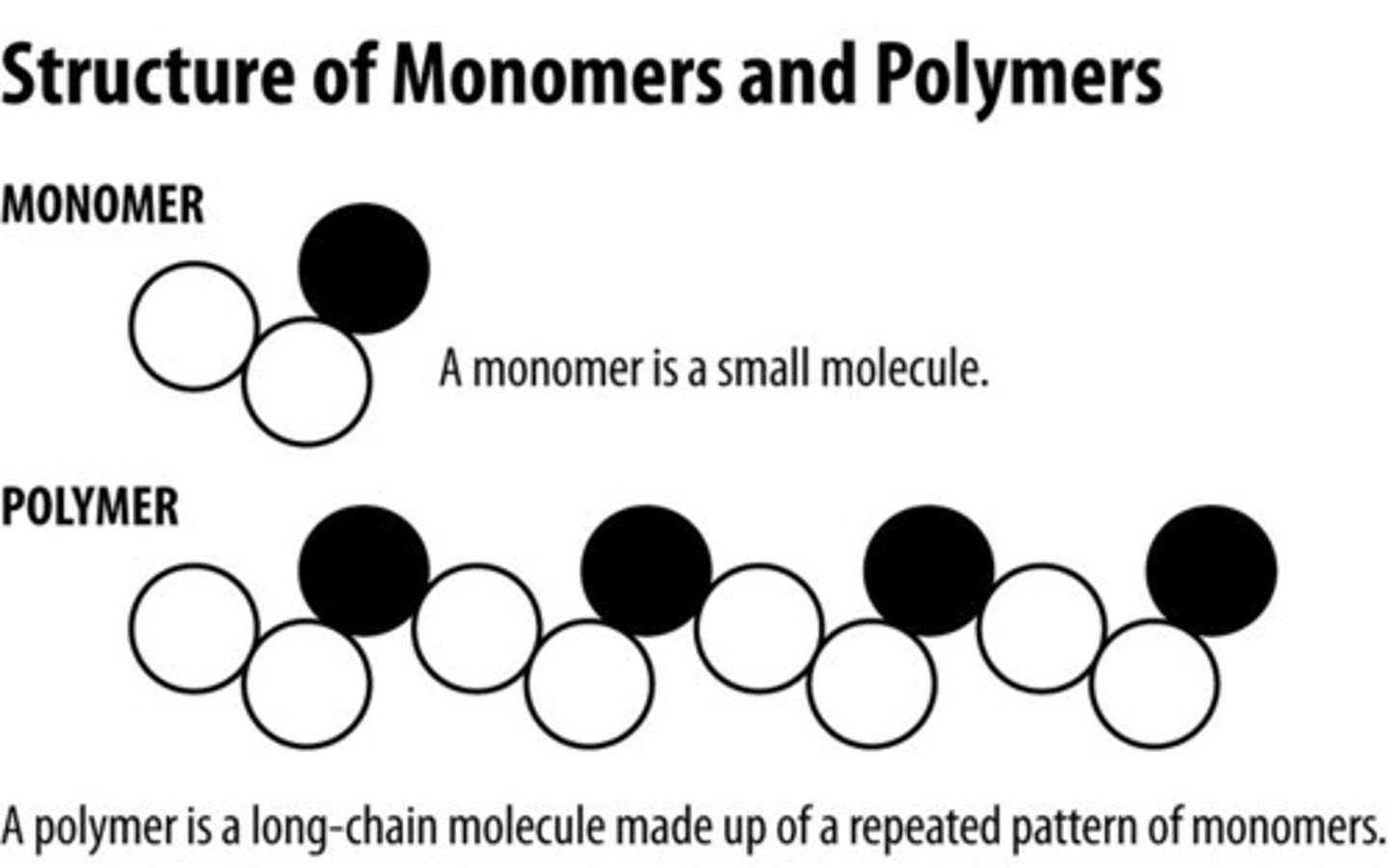
polymer
large molecule consisting of similar subunits called monomers
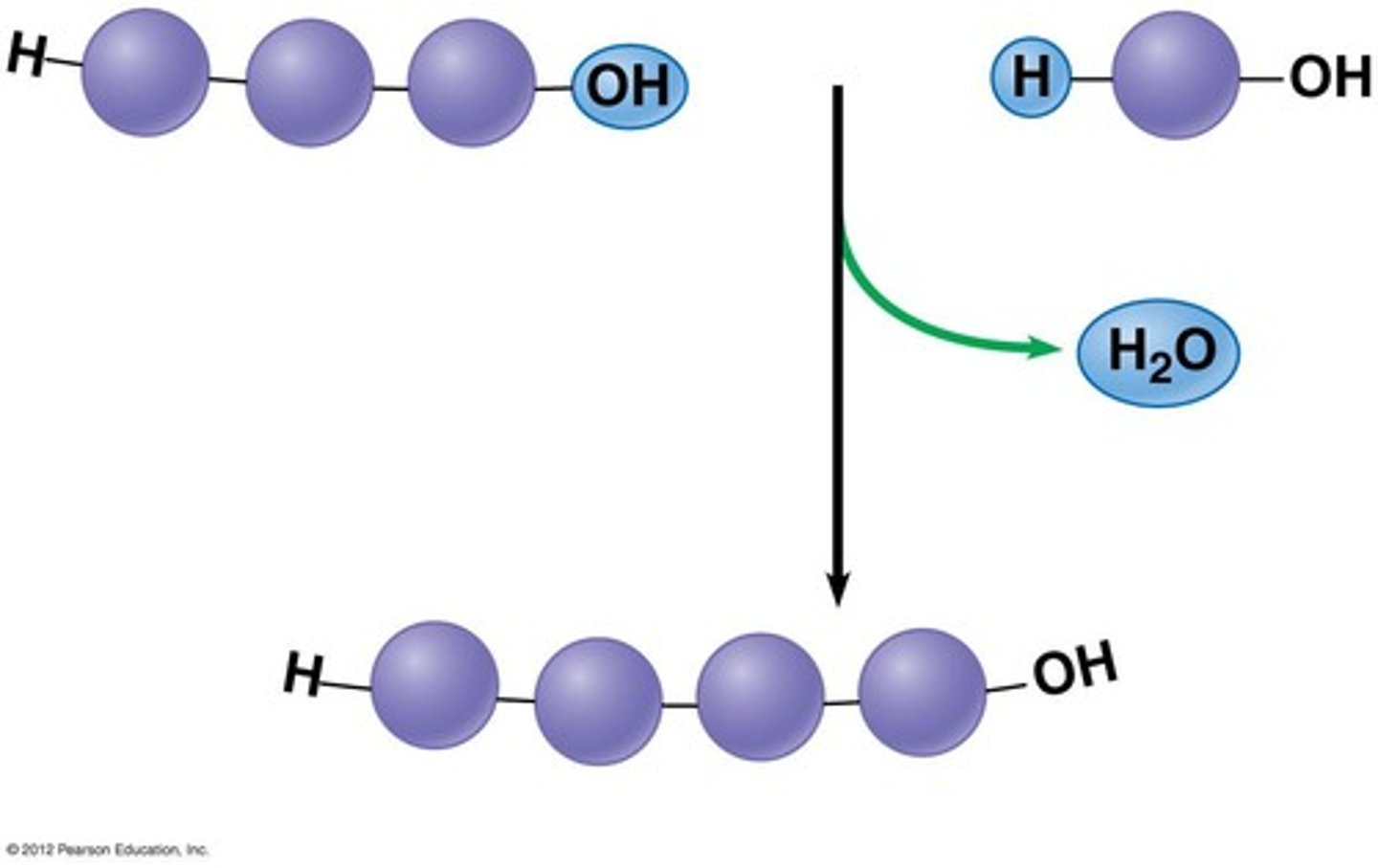
dehydration synthesis
water is removed to form a bond. Polymers are synthesized this way.
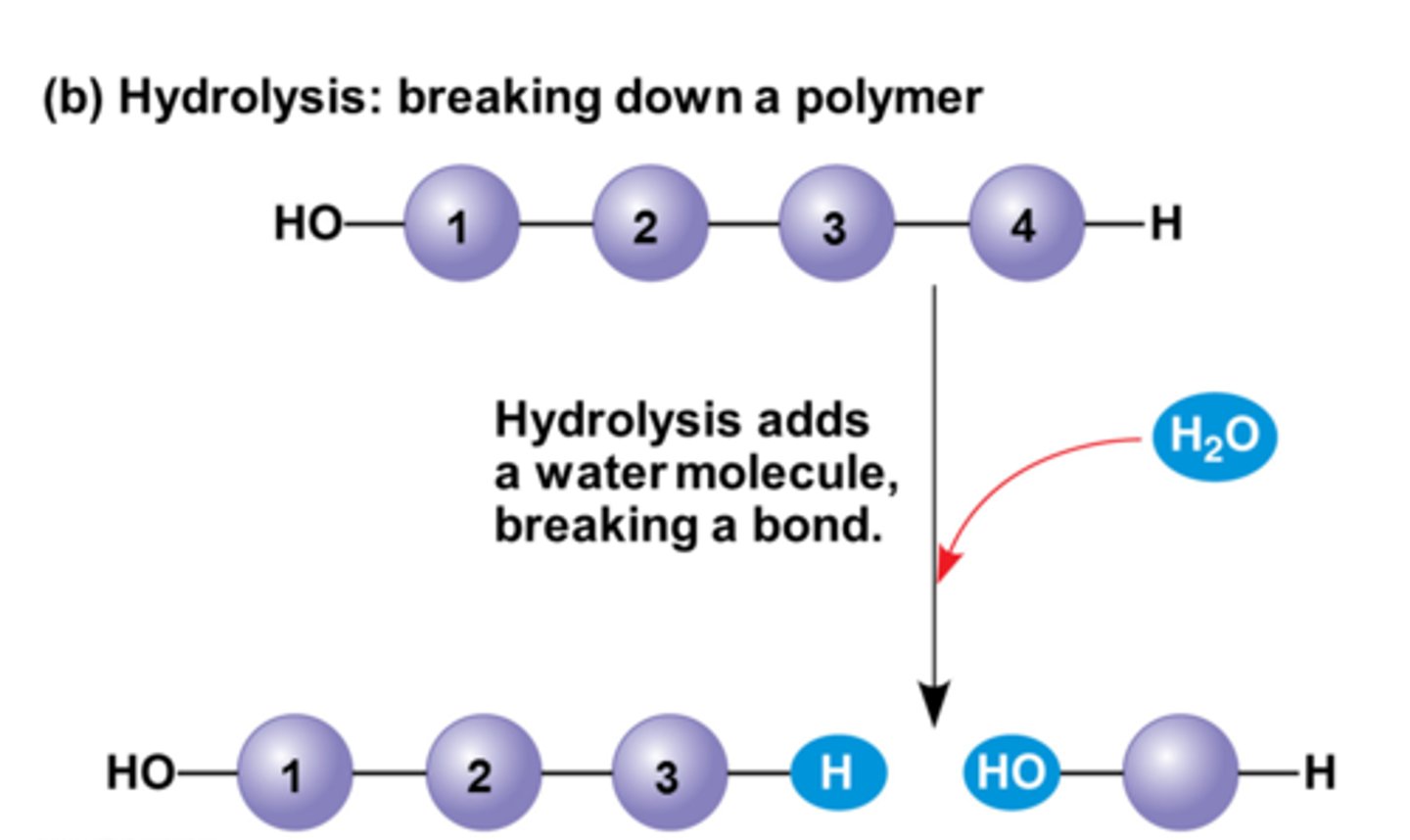
hydrolysis
water is added to break a bond. Polymers are broken down this way.
carbohydrates
sugars and starches; contain C, H, and O [(CH20)n]
![<p>sugars and starches; contain C, H, and O [(CH20)n]</p>](https://knowt-user-attachments.s3.amazonaws.com/07751725-cf21-467b-b2b8-f3a2ebb162bf.jpg)
what are the classes of carbohydrates?
monosacchrides, disaccharides, and polysaccharides
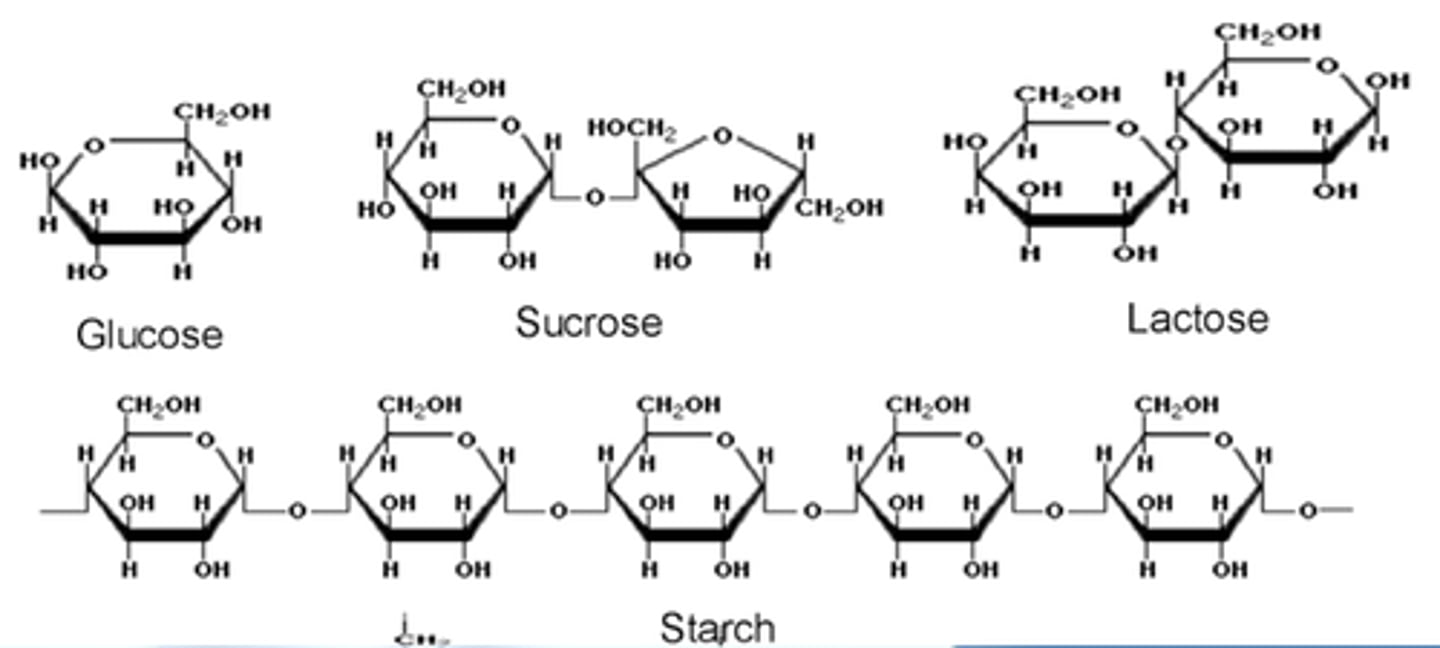
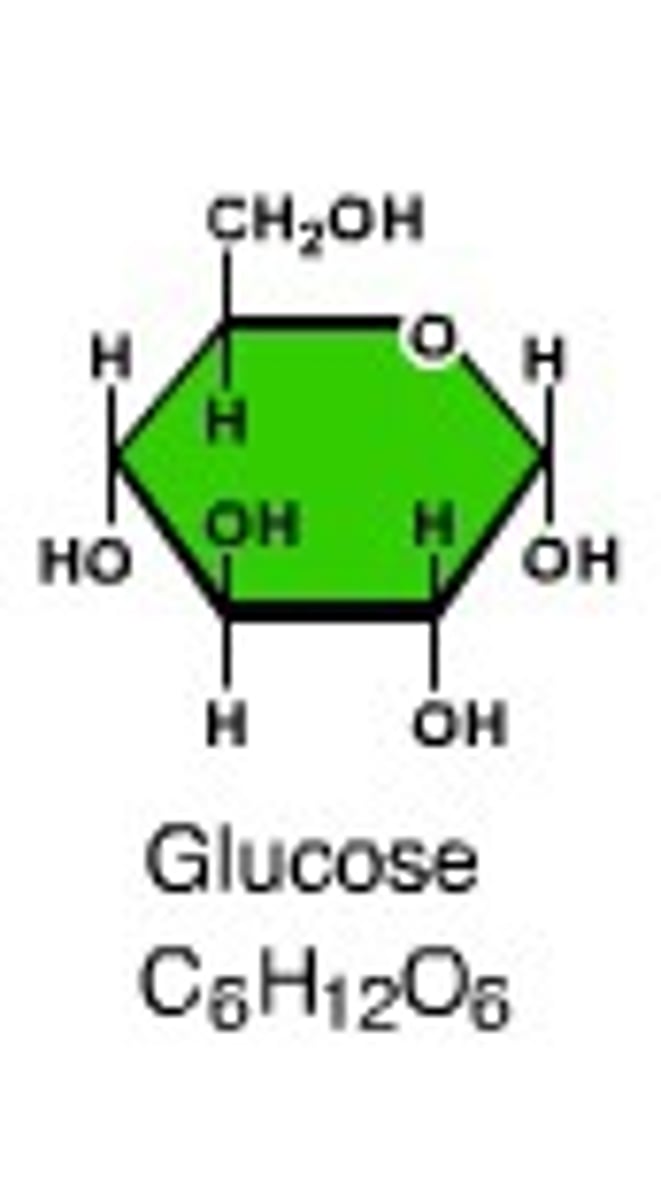
monosaccharides
glucose, fructose, galactose; building blocks of polysaccharides
disaccharides
sucrose, maltose, lactose; two monosaccharides bonded together
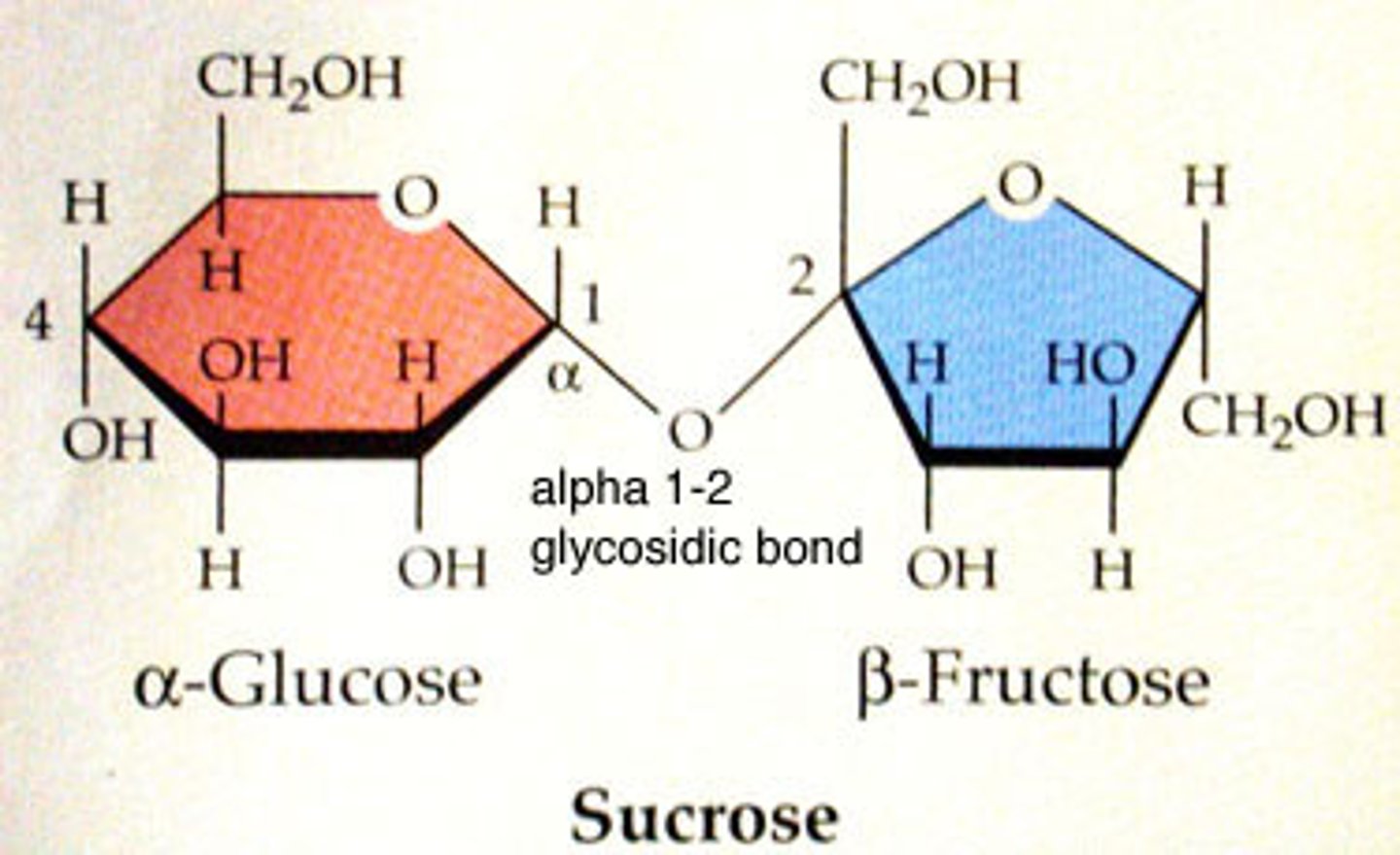
major source of cellular fuel (Ex: glucose) and structural molecules (Ex: ribose of RNA)
functions of carbohydrates
storage forms of carbohydrates
glycogen (animals), starch and cellulose/fiber (plants)
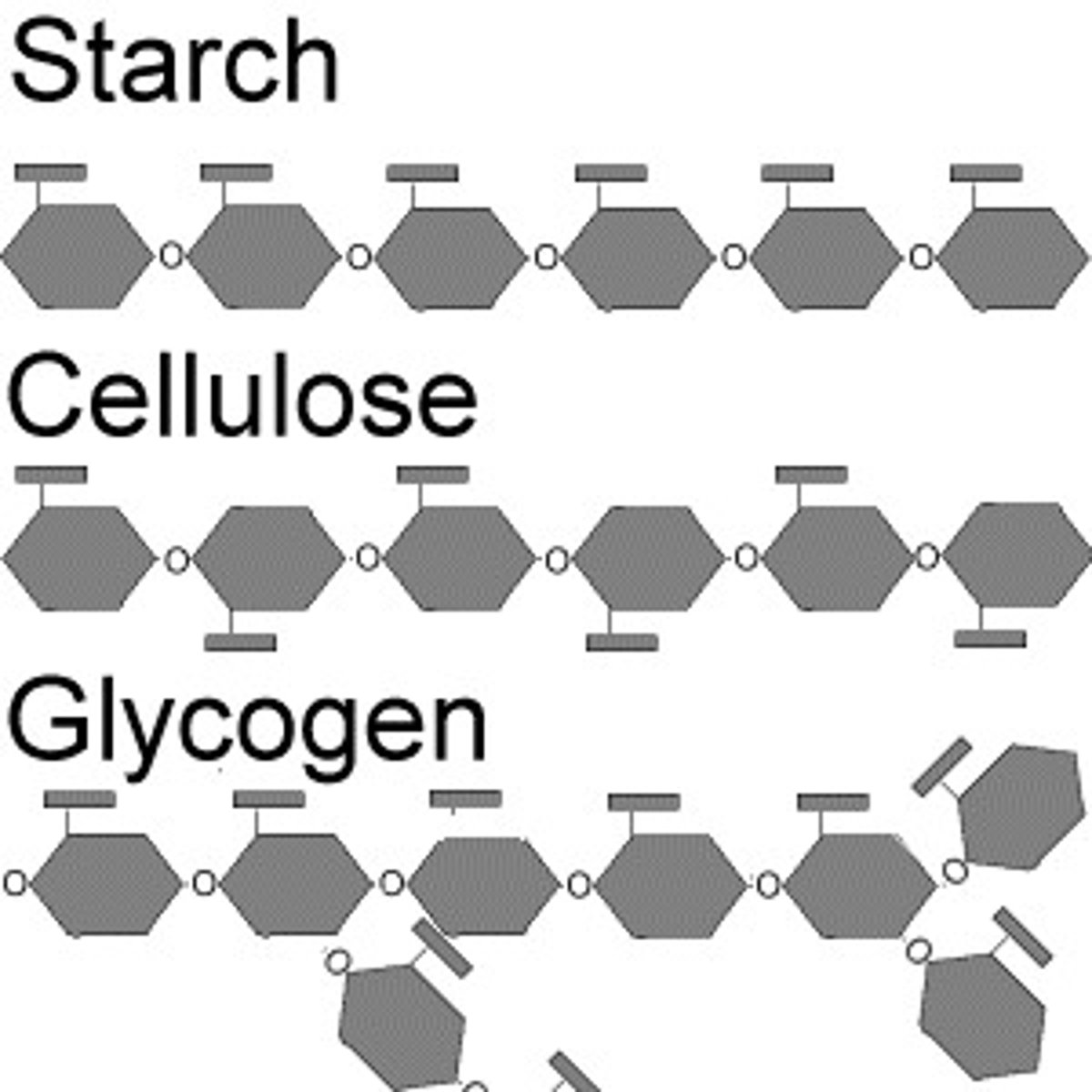
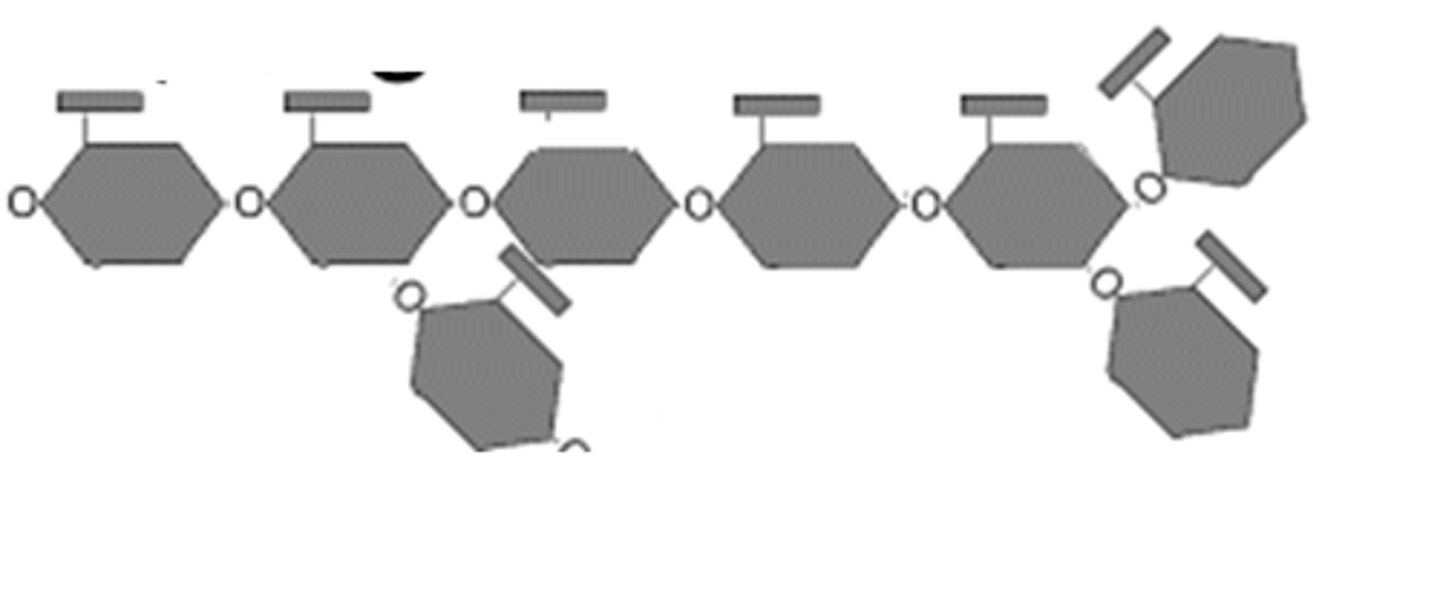
glycogen
polysacchrides that animal cells use to store sugar
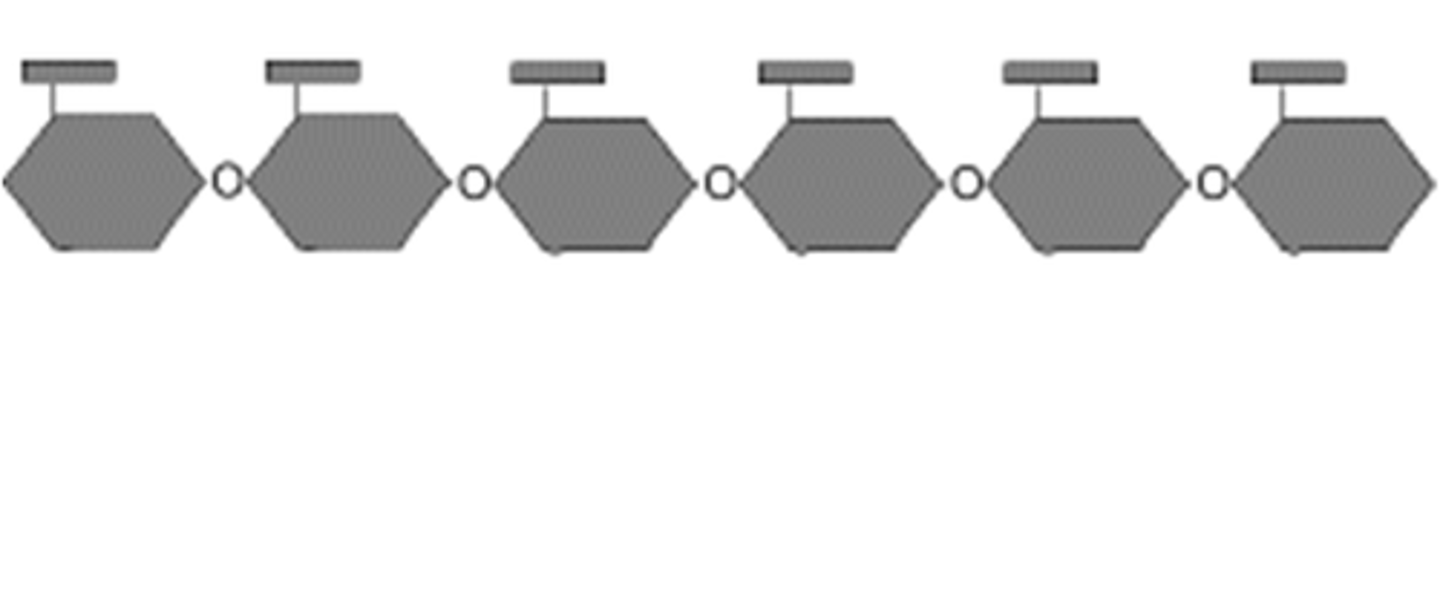
starch
storage polysacchrides used by plants (Potatoes, rice)
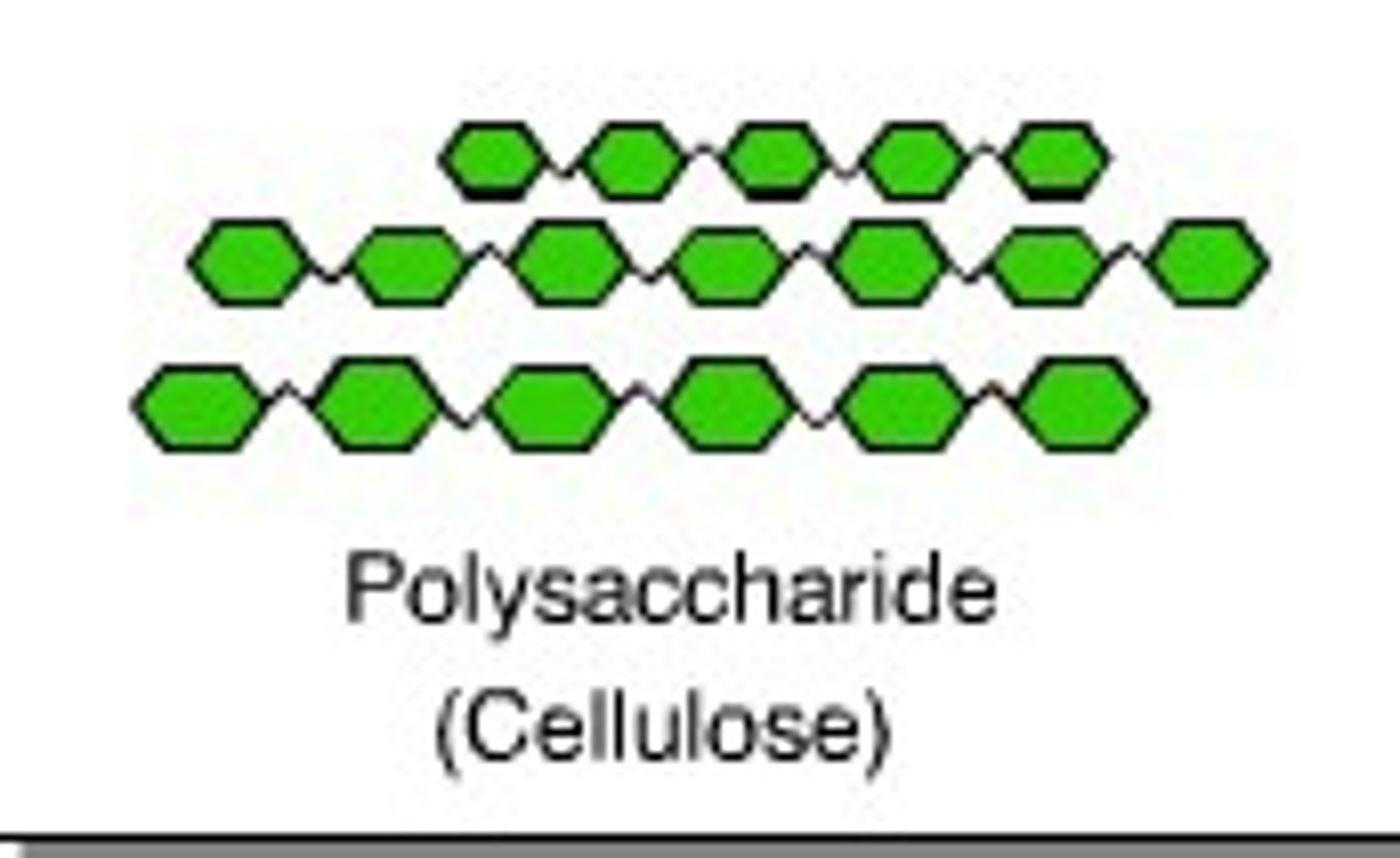
fiber (cellulose)
polysaccharide found in all plants that are undigestible for humans but necessary for a healthy dietary tract
lipids
insoluble in water but dissolve in other lipids; all lipids are non-polar
types of lipids
triglycerides, neutral fats, phospholipids, steriods (cholestrol), and eicosanoids
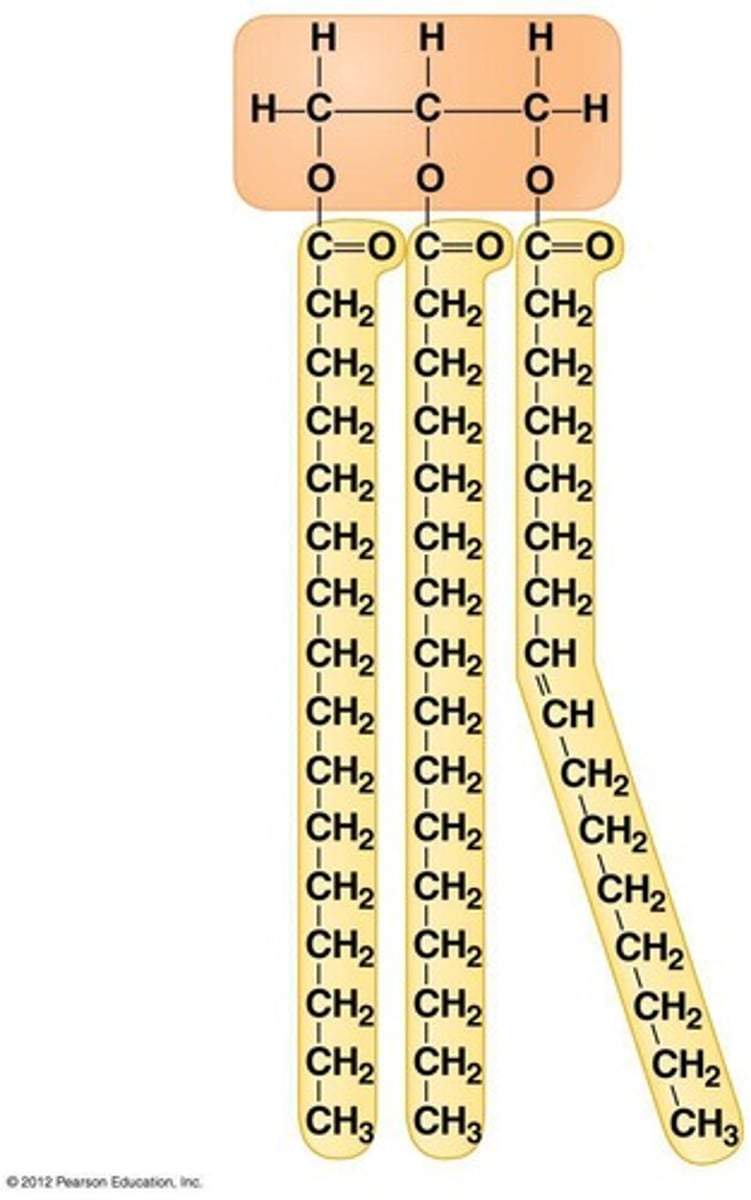
triglycerides
neutral fats; composed of three fatty acids bonded to a glycerol molecule
main functions of lipids
Energy storage, insulation, protection
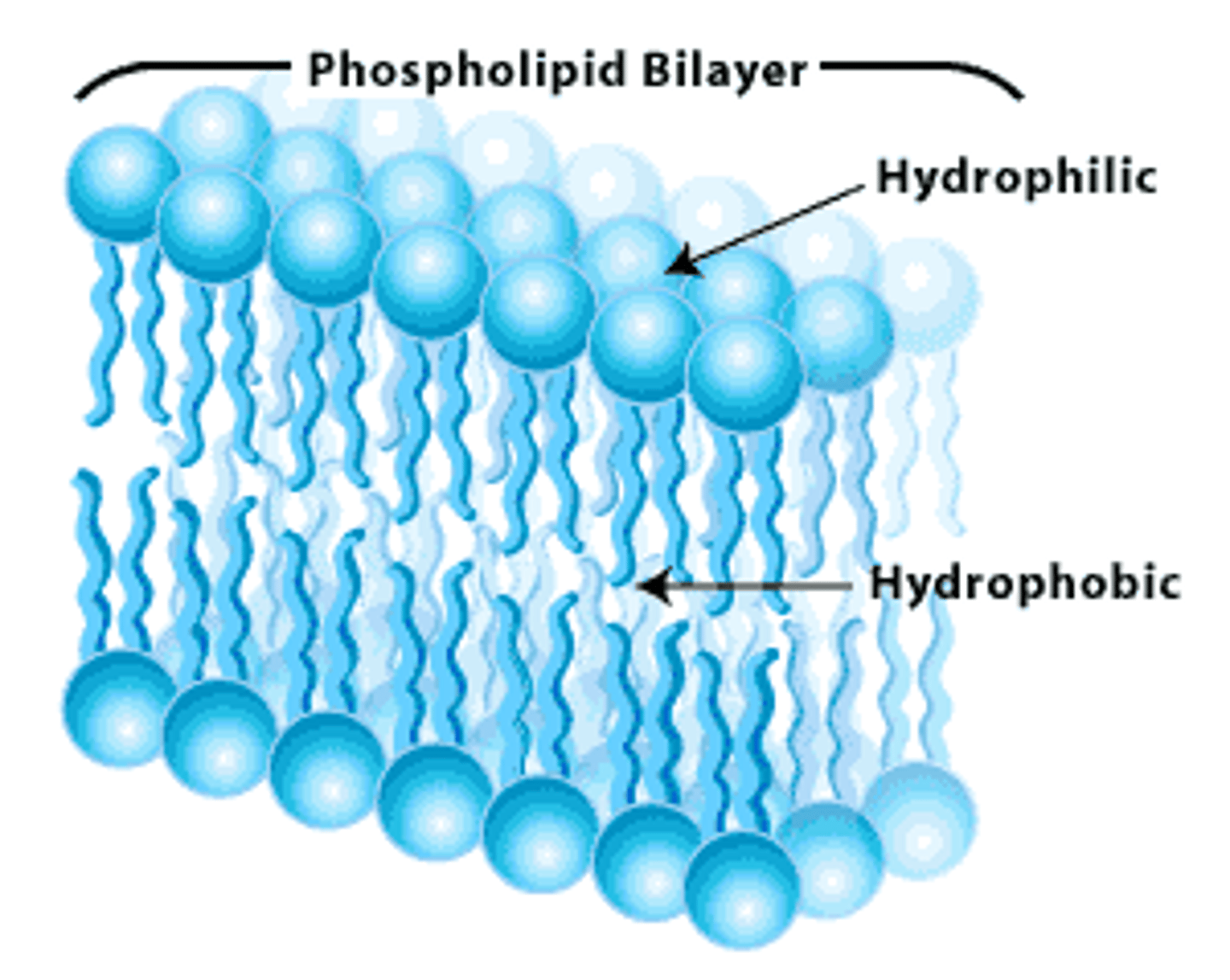
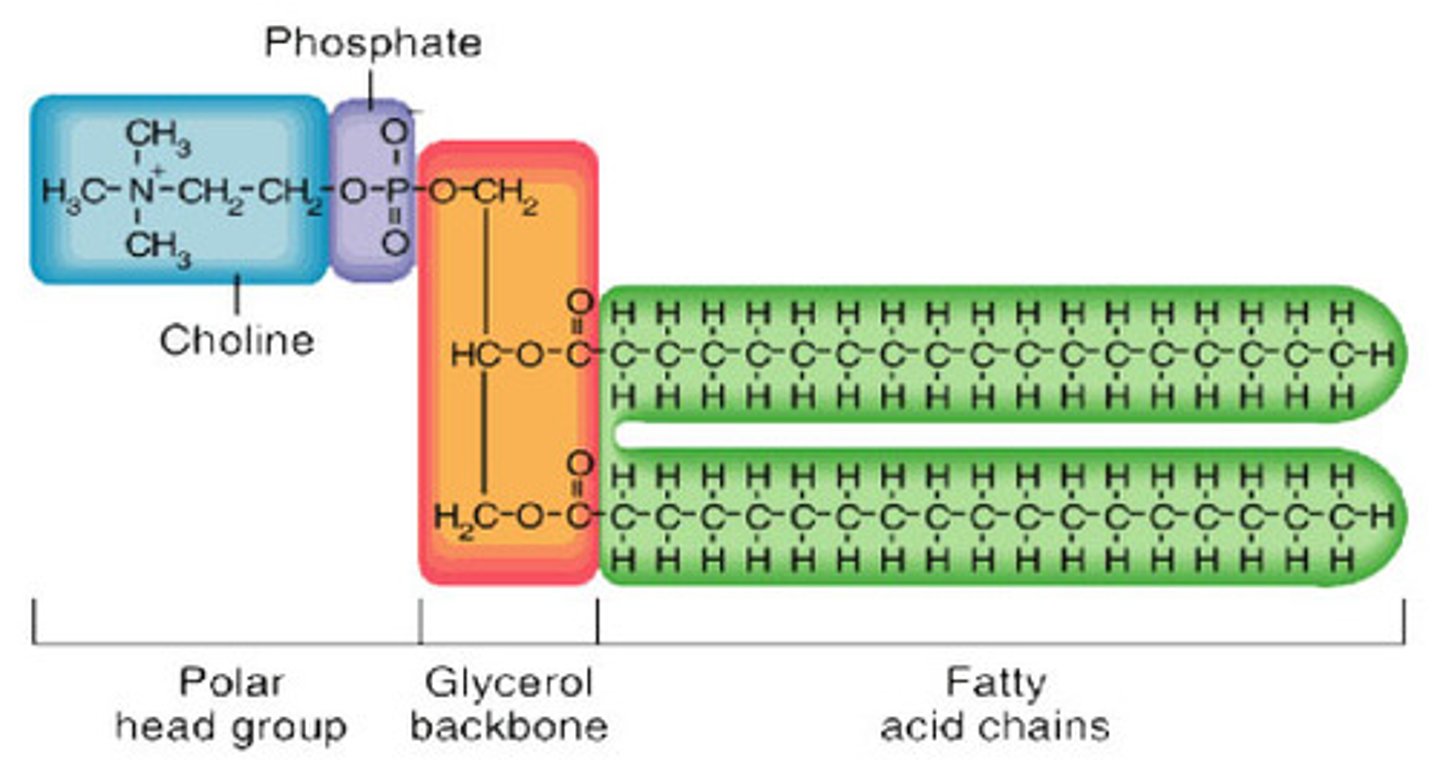
phospholipids
head (polar/hydrophilic) and tail (nonpolar/hydrophobic) regions that have different properties making the molecule amphiphilic; major component of cell membranes
steroids
An interlocking 4 ring structure including cholestrol, vitamin D, steriod hormones (estrogen, testosterone, cortisol) and bile salts.
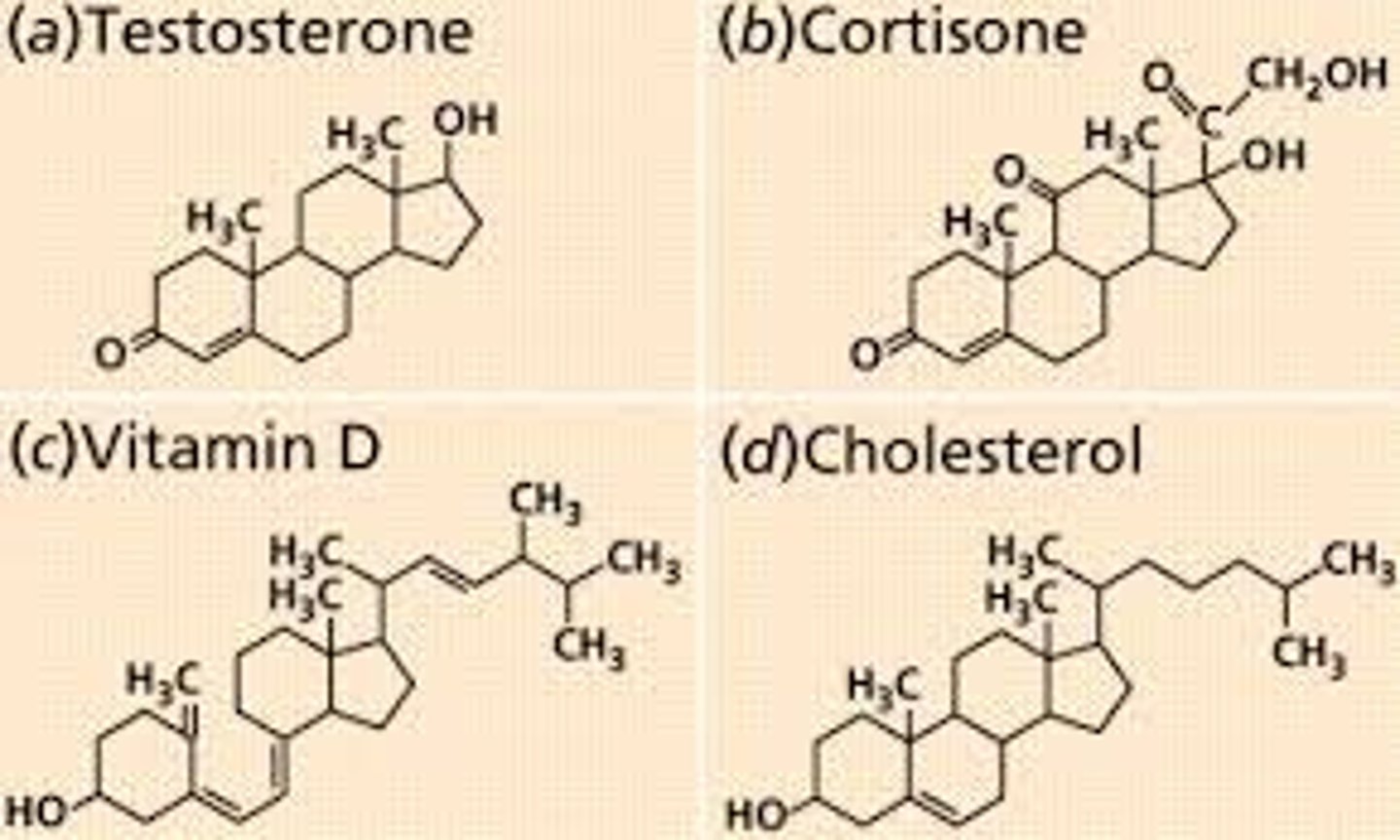
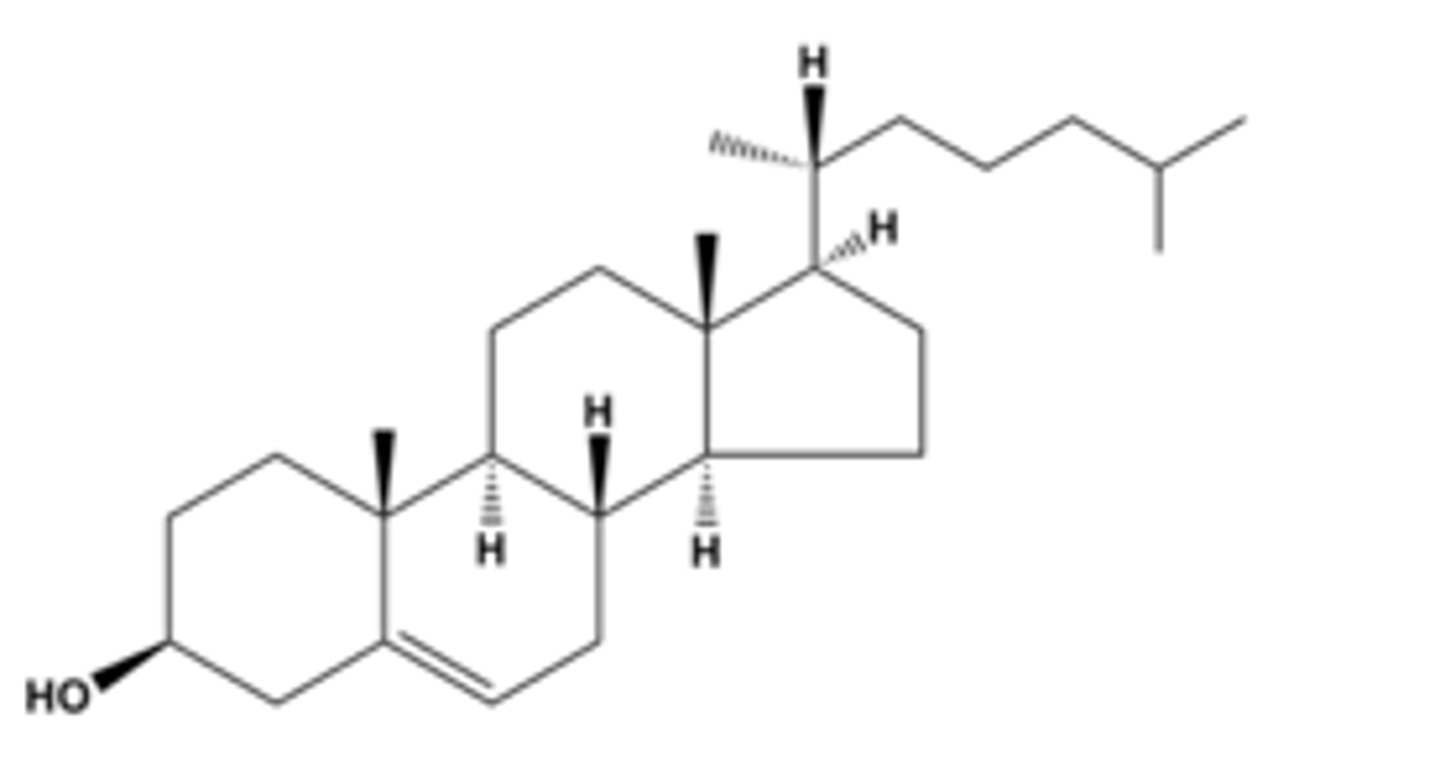
cholesterol
most important steriod; basis for all steriods formed in the body.
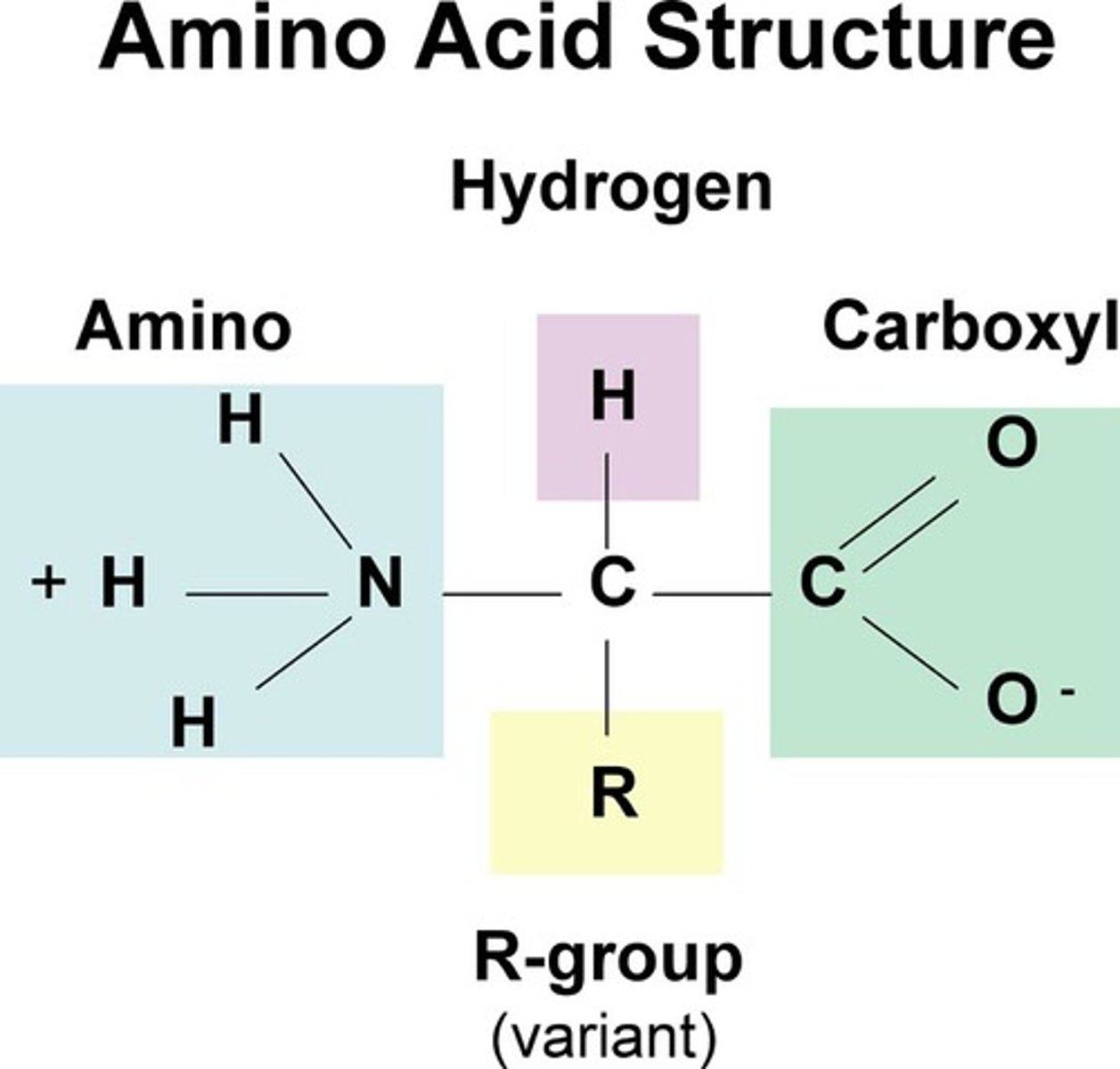
proteins
polymers of amino acids
amino acids
monomers of proteins; joined by covalent peptide bonds
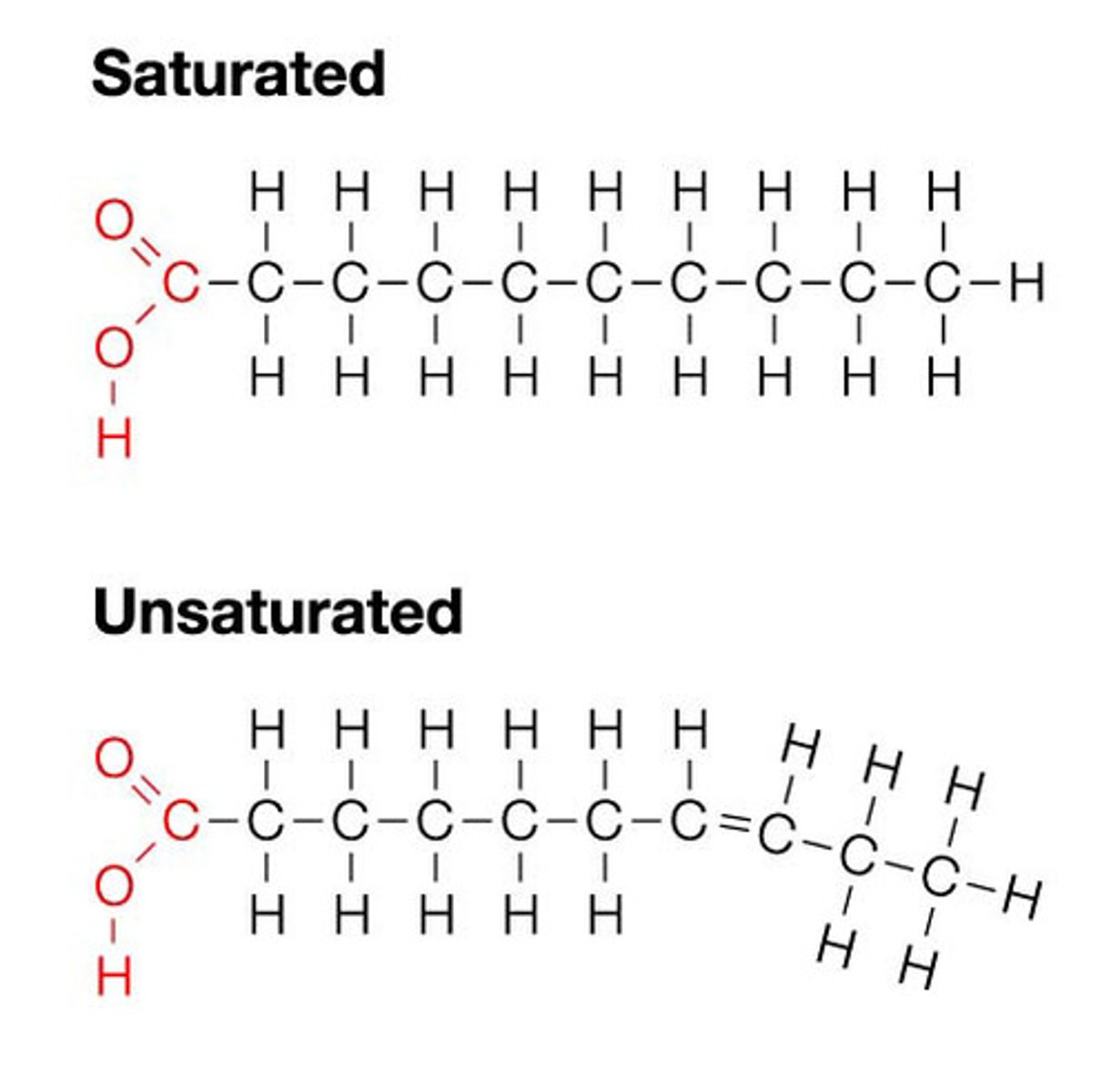
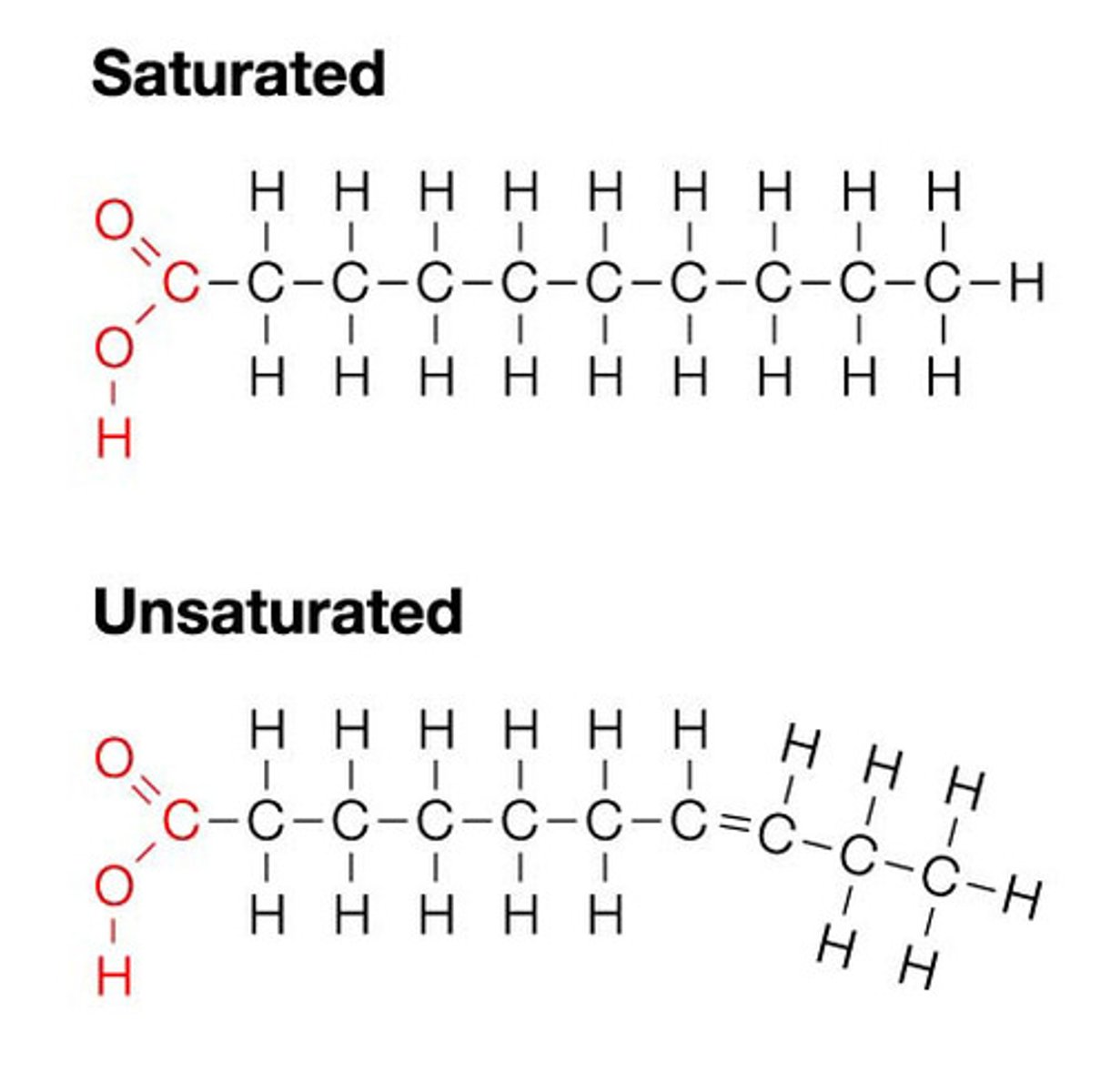
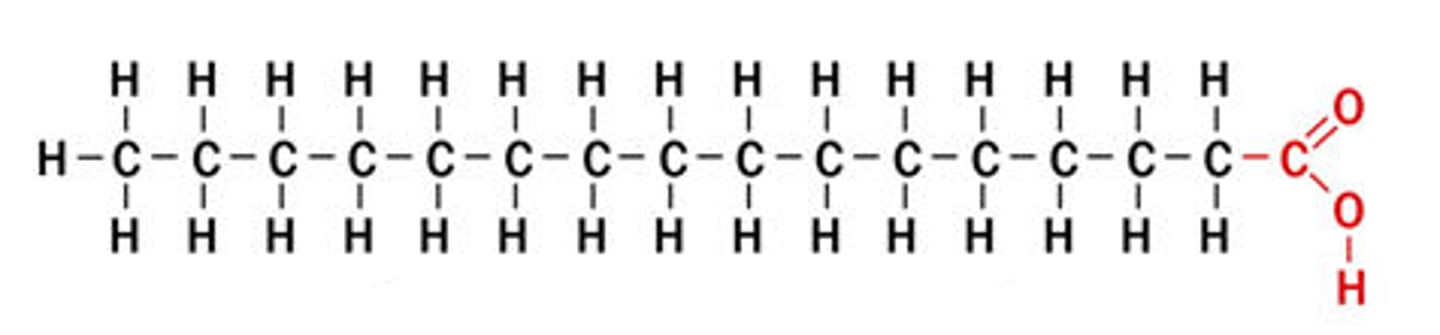
saturated fatty acids
single covalent bonds between C atoms; max number of H atoms; solid animal fats (butter)
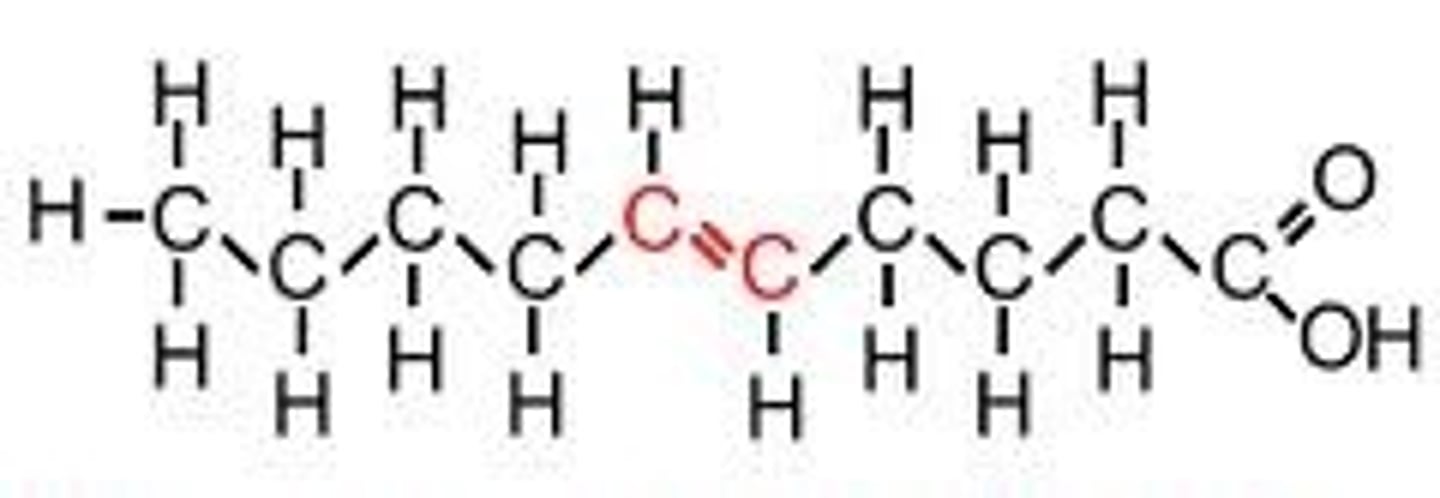
unsaturated fatty acids
one or more double bonds between C atoms; reduced # of H atoms; heart healthy; plant oils (olive oil)
levels of protein structure (4)
Primary, Secondary, Tertiary, and Quaternary.
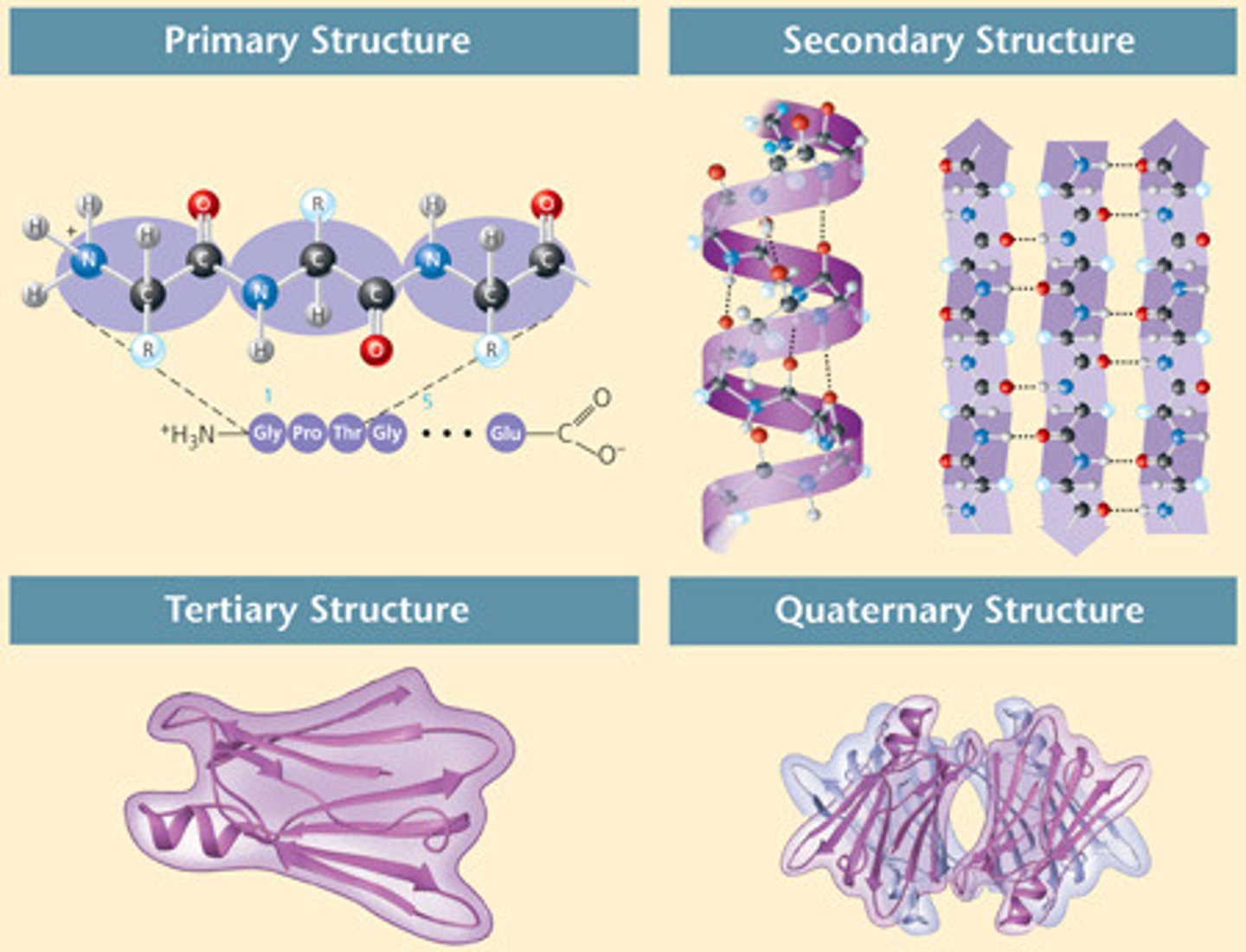
primary structure
sequence of amino acids forms the polypeptide chain
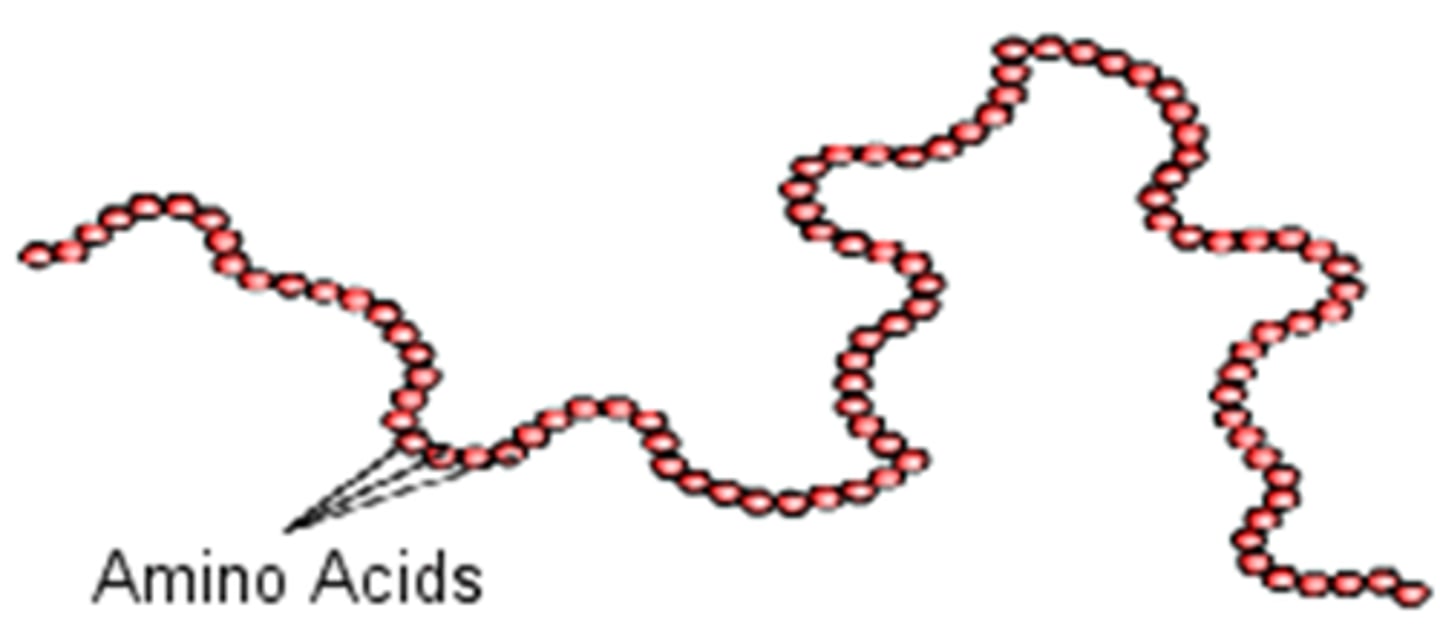
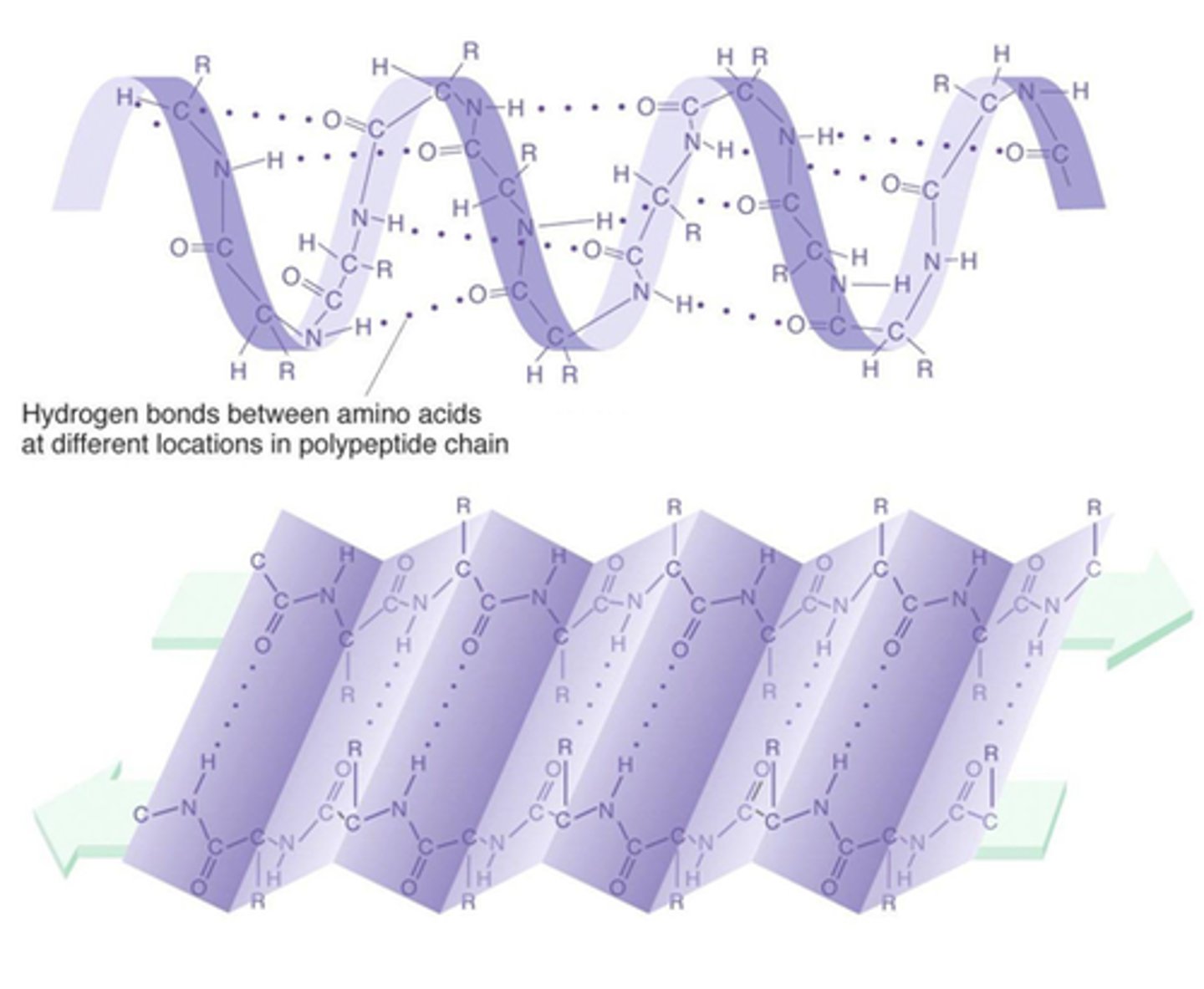
secondary structure
primary chain forms spirals (a-helices) and (beta) sheets
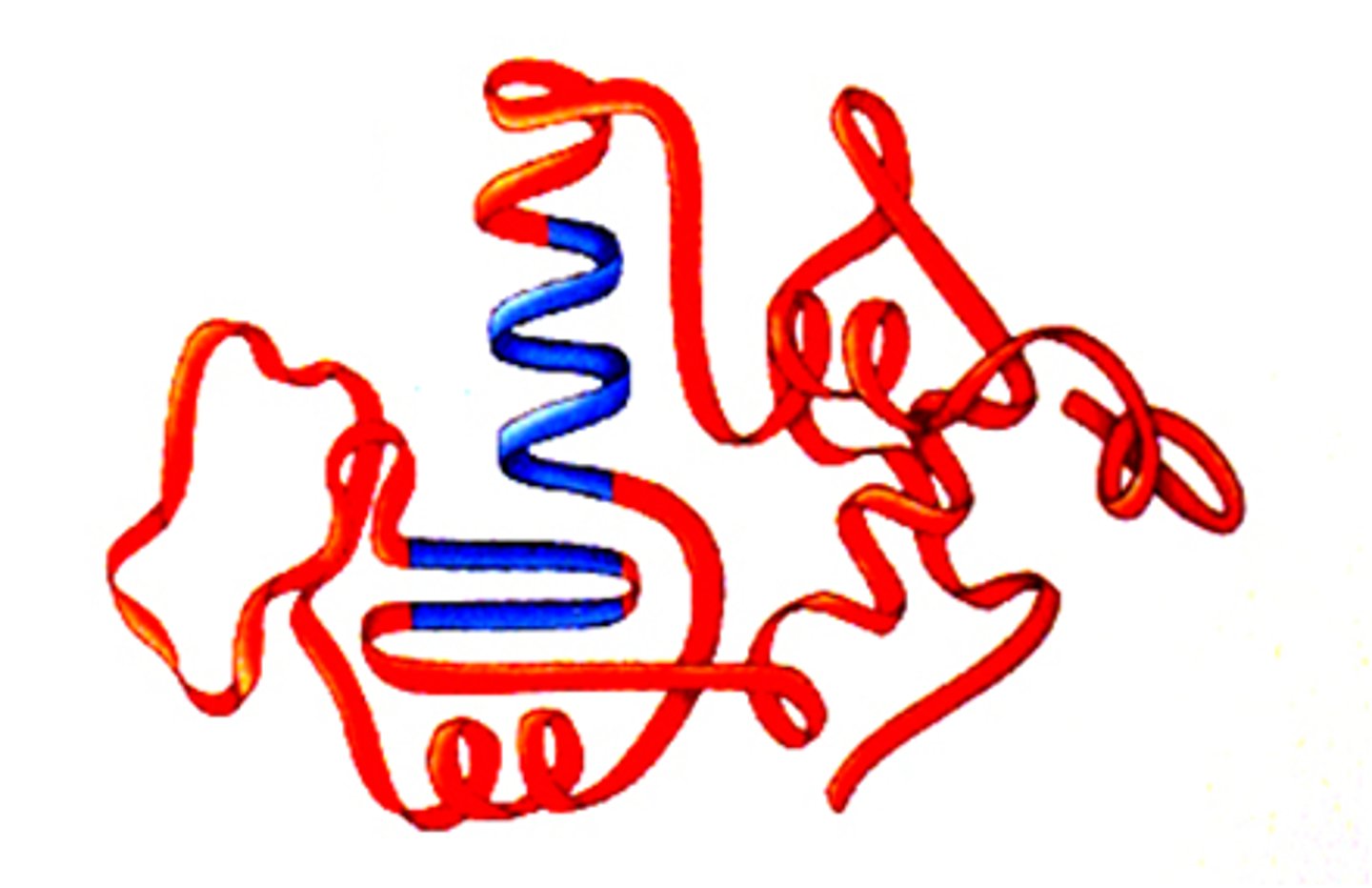
tertiary structure
superimposed on secondary structure.
-Helices and/or -sheets are folded up
to form a compact globular molecule
held together by intramolecular bonds
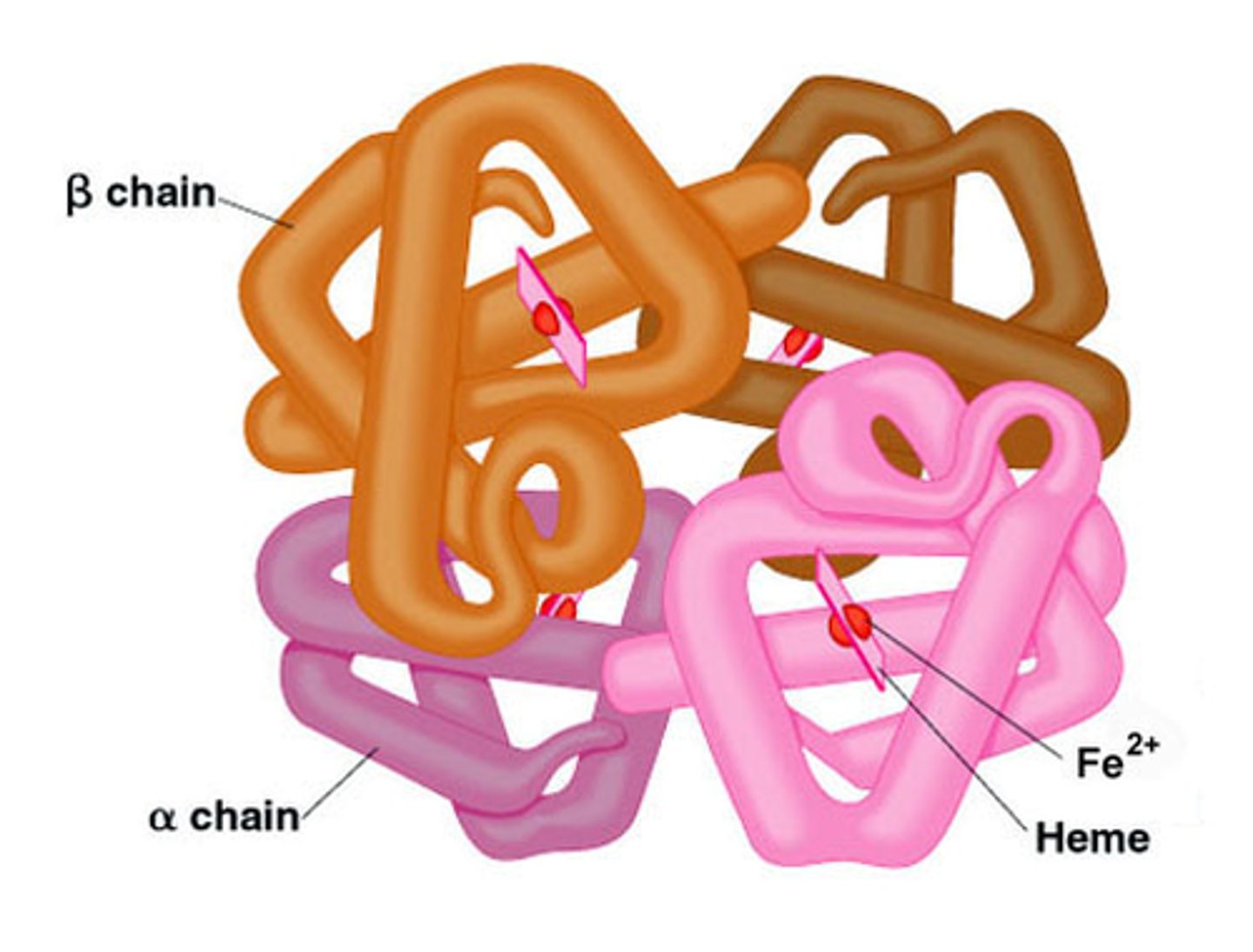
quaternary structure
two or more polypeptide chains,
each with its own tertiary structure,
combine to form a functional
protein
Protein denaturation
proteins unfold and lose functional 3-D shape; active sites destroyed (cannot function); usually reversible if normal conditions restored
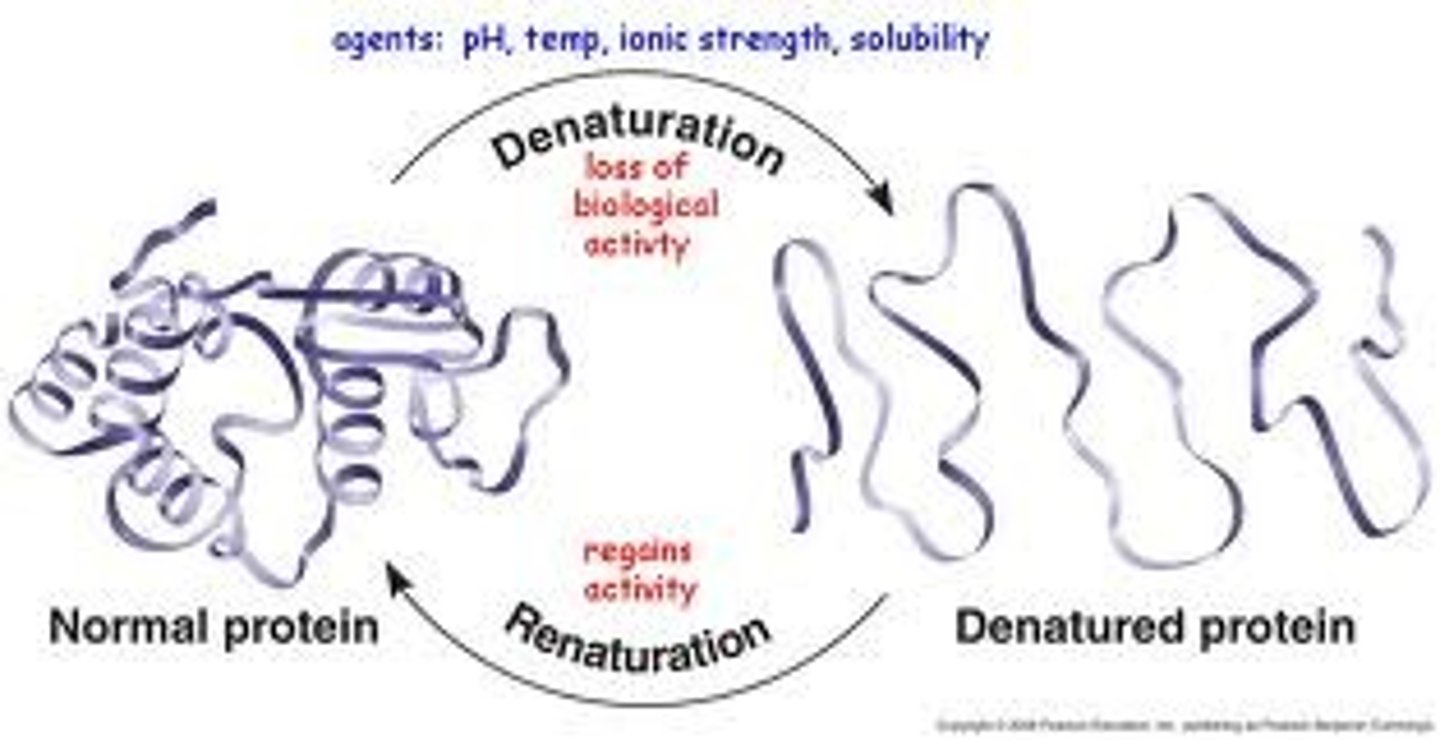
what causes protein denaturation?
can be caused by decreased pH or increased temperature
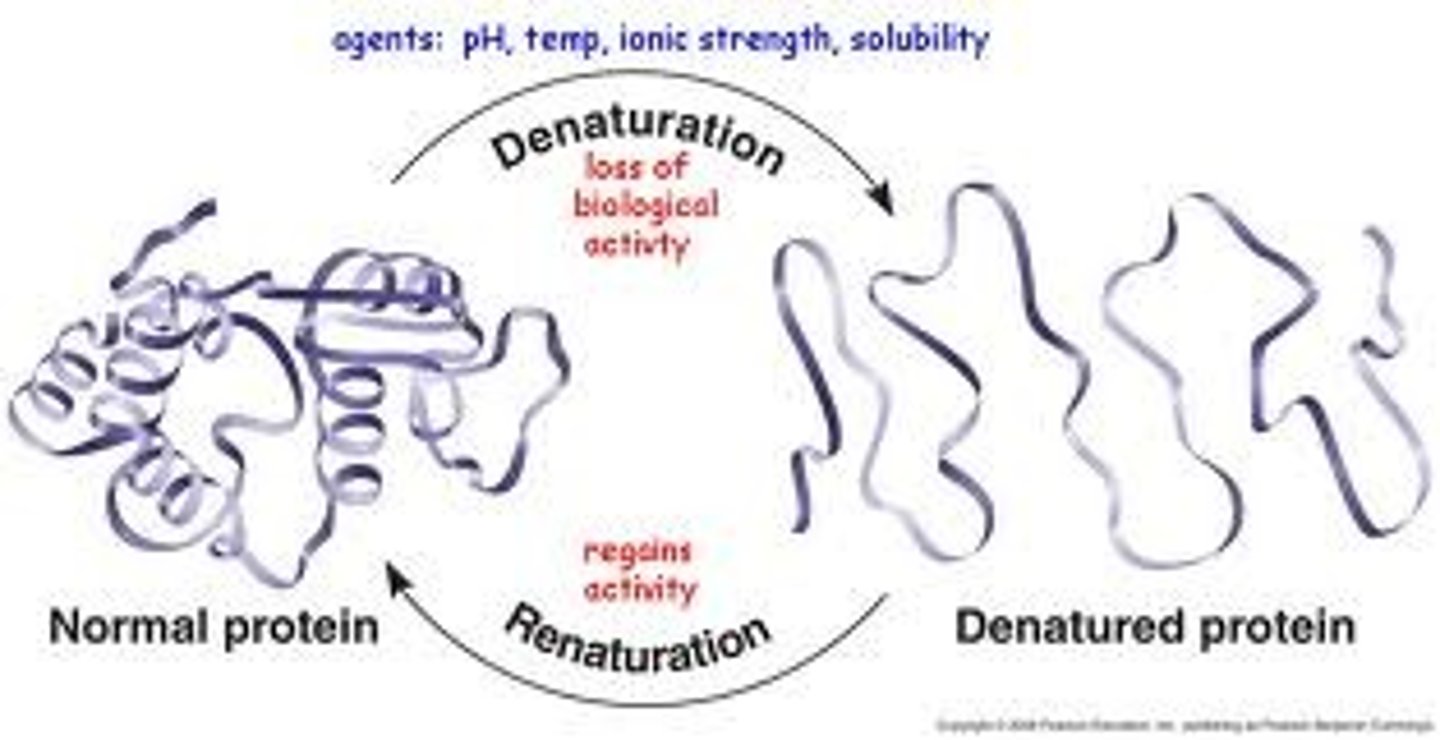
cofactor/coenzyme
component required to make certain enzymes functional; metal ion (cofactor) - organic molecule often a vitamin (coenzyme)
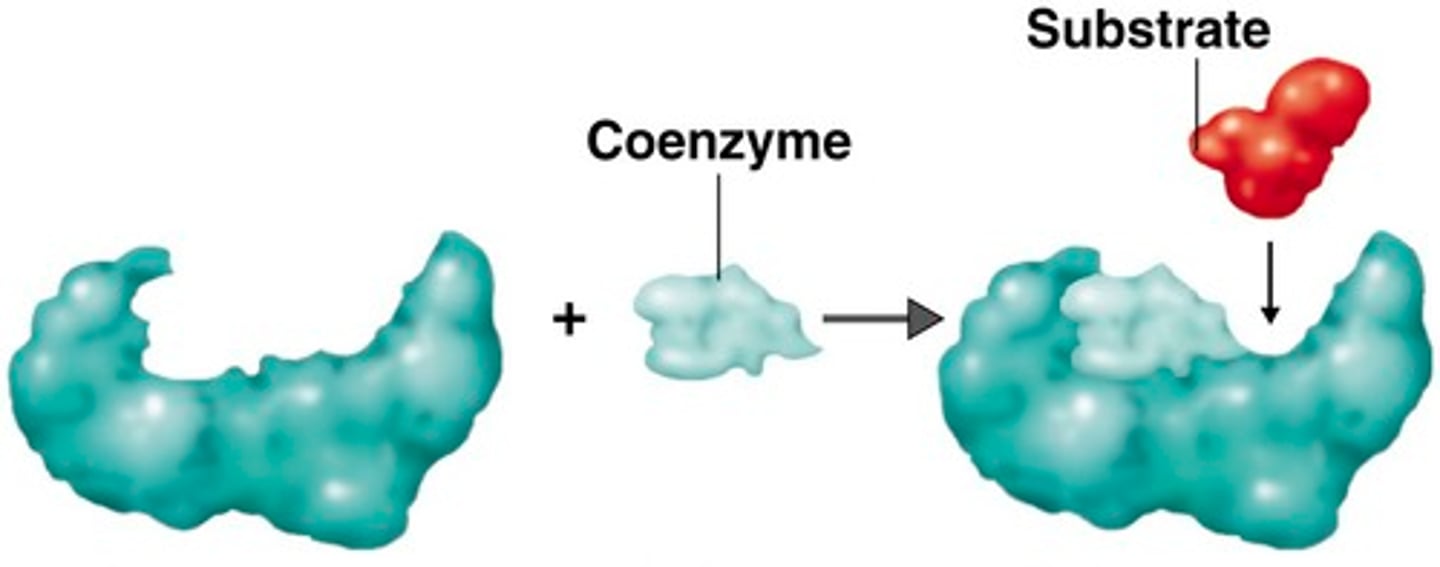
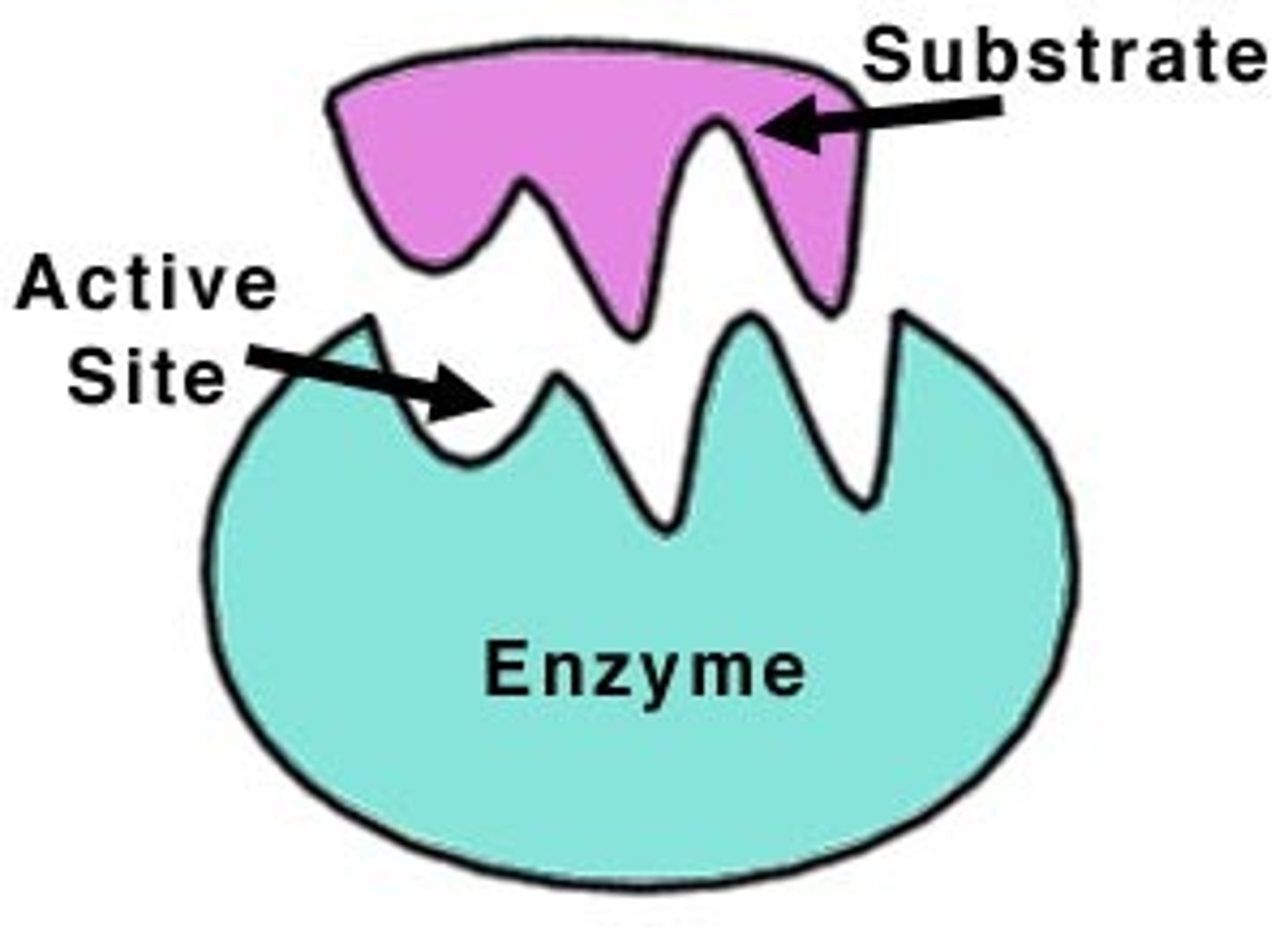
enzymes
proteins that act as biological catalysts (regulate and increase speed of chemical reactions by lowering activation energy)
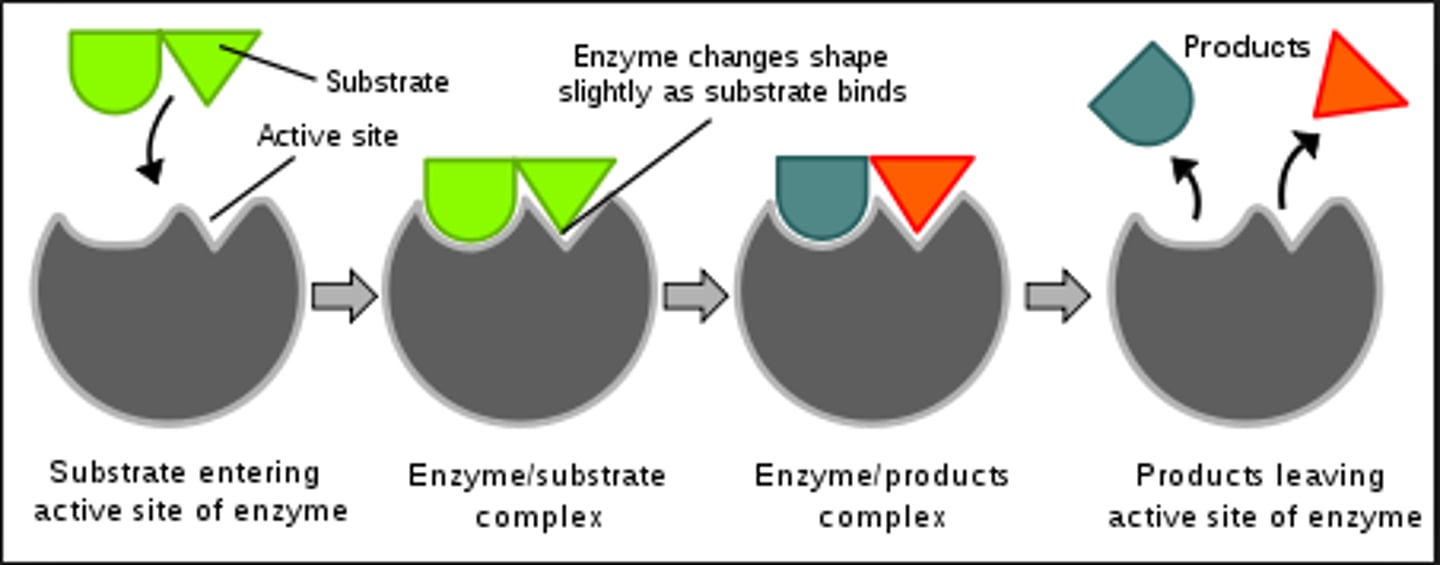
enzyme characteristics
some functional enzymes consist of two parts (protein and cofactor/coenzyme); enzymes are specific; usually end in -ase; often named for the reaction they catalyze (Ex: hydrolase, sucrase)
nucleic acids
ribonucleic acid (RNA) and deoxyribnucleic acid (DNA); largest molecules in the body
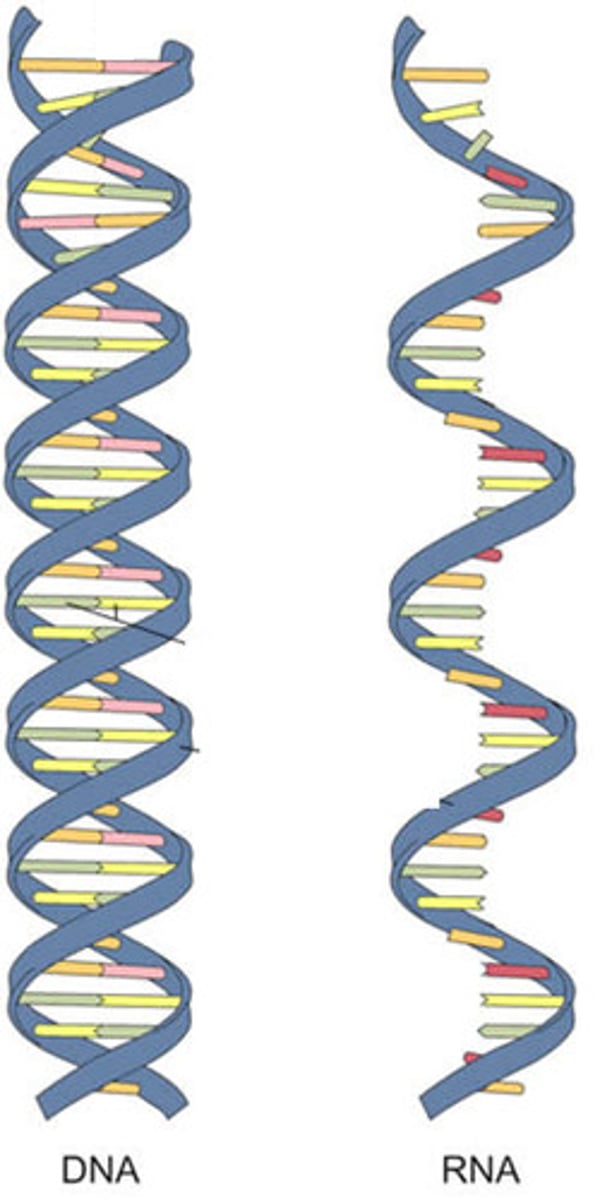
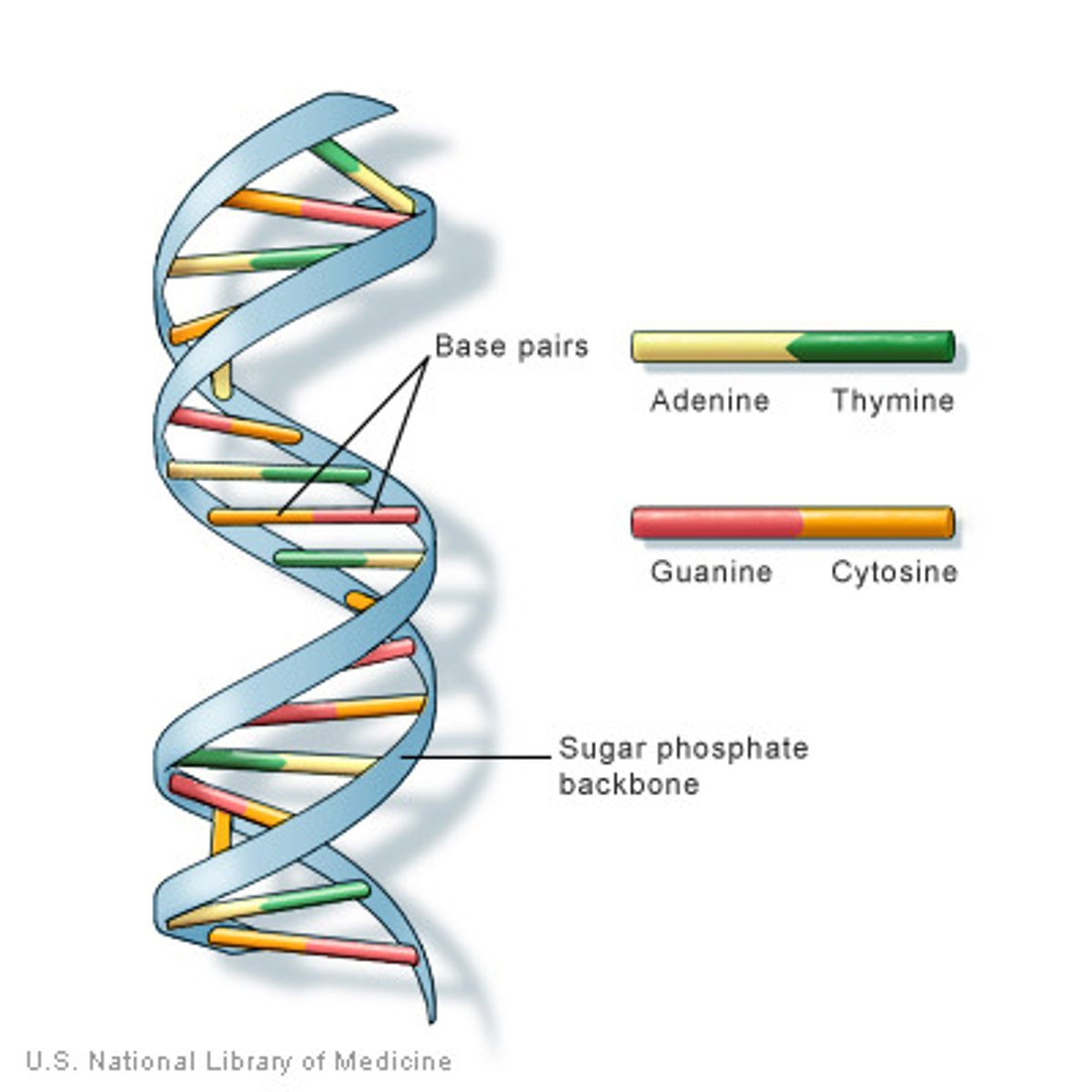
deoxyribonucleic acid (DNA)
double-stranded helical molecules (double helix) located in the cell nucleus; provides instructions for protein synthesis
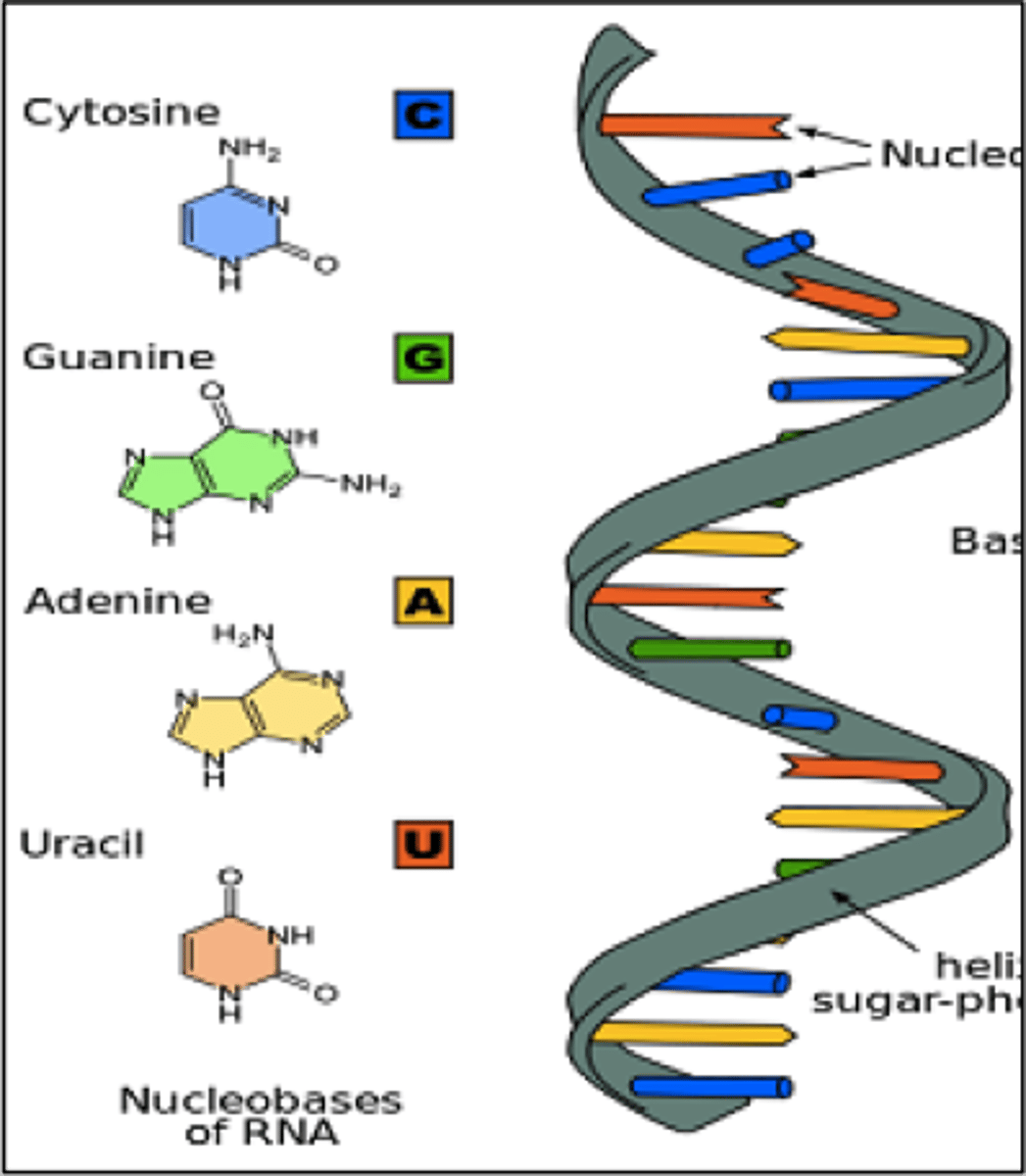
ribonucleic acid (RNA)
single-stranded molecule mostly active outside the nucleus; carry out the DNA orders for protein synthesis
adenosine triphosphate (ATP)
directly powers chemical reactions in cells; energy form immediately useable by all body cells
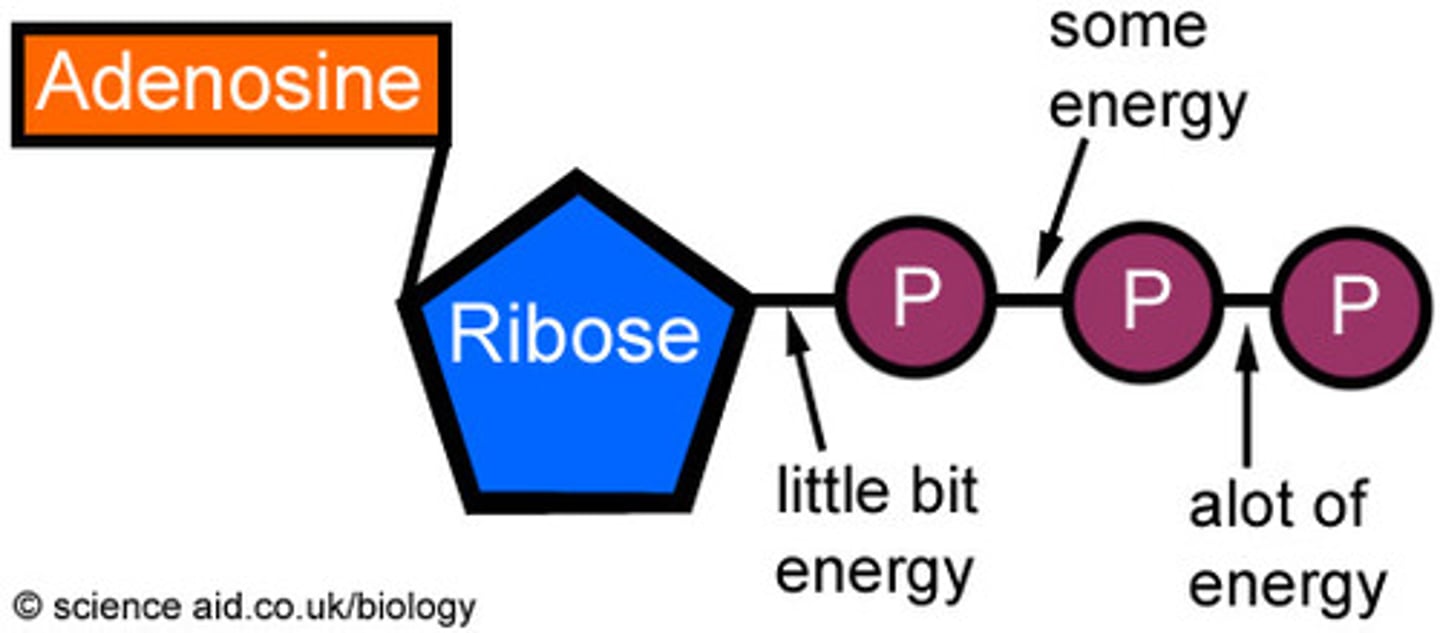
where does the cell get the energy that is stored in ATP?
breaking the chemical energy in bonds of fuels (carbohydrates, proteins, fats) then storing the energy in ATP
what are some examples of cellular work driven by ATP?
transport work, mechanical work, chemical work