Hematology 13: Anemia Part 1 (Microcytic-Hypochromic)
1/63
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
64 Terms
Morphologic Classification Chart
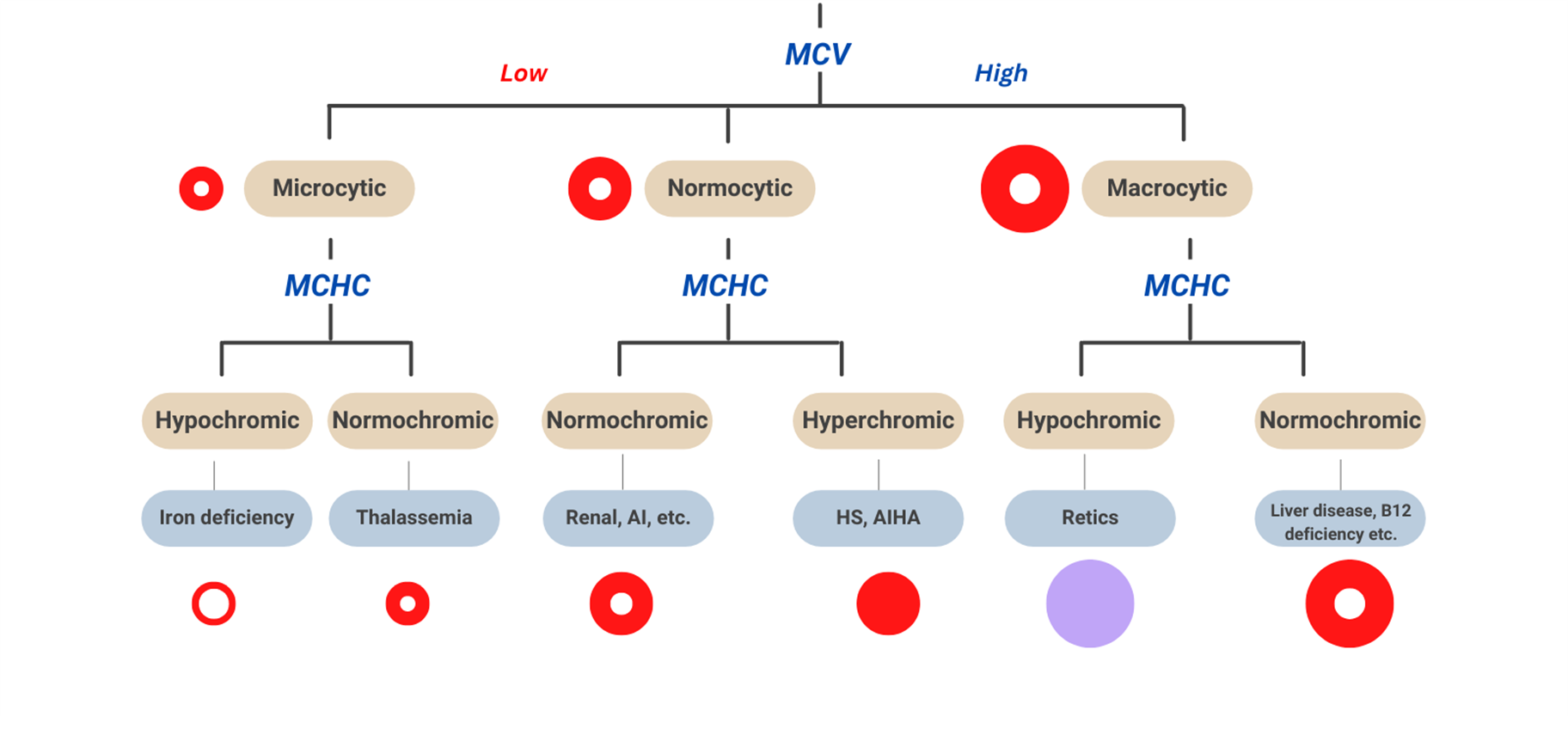
Microcytic-Hypochromic Anemia
circulating RBCs are smaller than the usual size of RBCs (microcytic) and have decreased red color (hypochromic), most common anemia, result from impaired hemoglobin synthesis
Hgb < 12 g/dL (F) or < 13 g/dL (M) with mean cell volume (MCV) < 80 fL
common causes of Microcytic-Hypochromic Anemia
Blood loss
Iron-deficiency anemia (IDA)
Thalassemia
Anemia of chronic disease (ACD)
Sideroblastic anemia
Lead poisoning
Etiology: Blood loss
Acute
: temporary anemia caused by a rapid hemorrhage due to trauma (e.g., car accident, surgery, or childbirth).
The body quickly replaces plasma but usually needs several weeks to replace RBCs
Etiology: Blood loss
chronic
Chronic: anemia develops due to lack of sufficient functional iron and hemoglobin-deficient RBCs (e.g., GI bleeding, heavy menstruation)
pathophysiology for iron deficiency anemia
Iron loss exceeds iron intake
clinical presentation of blood loss leading to ida
Fatigue, pallor, shortness of breath
Laboratory Evaluation of Blood Loss
Peripheral blood smear
MCV 55-74 fL – microcytic
MCHC 22-31 g/dL – hypochromic
Reticulocyte count
High
Iron deficiency anemia (IDA)
is a common form of anemia that occurs when the body has insufficient iron to produce hemoglobin.
Iron deficiency is the most common nutritional deficiency in the world
Affects 8-10% of children in the US
Prevalent in countries where:
Grain major part of the diet, and meat is scarce
Parasitic infections are endemic (i.e., hookworm)
Defective Hgb production can be due to disturbances in:
Heme synthesis
Iron deficiency
Defective iron metabolism
Defective porphyrin synthesis
Globin synthesis
Result of defective Hgb production
Microcytic hypochromic anemia
does iron need to be bound to protein to do function?
yes
enterocytes
digestive cells that absorb iron
hepatocytes
liver cells store iron
macrophages
remove senescent rbc from spleen, bone marrow and liver
iron is primarily found in (5):
circulating rbc, enterocytes, hepatocytes, splenic macrophages and rbc precursors
where is most of iron found
hb
iron distribution
Hgb is the major fraction of body iron
Iron in Hgb remains in RBC until cell is removed from circulation.
Splenic macrophages degrade and release Hgb
Iron, bound to transferrin, is recycled for heme synthesis in BM
absorption - Iron in the enterocyte can be:
stored as ferritin, then transported via ferroportin
when does intestinal iron absorption increase?
when erythropoietin increases
when absorbed in the blood, what is iron bound to?
transferrin
iron cycle diagram
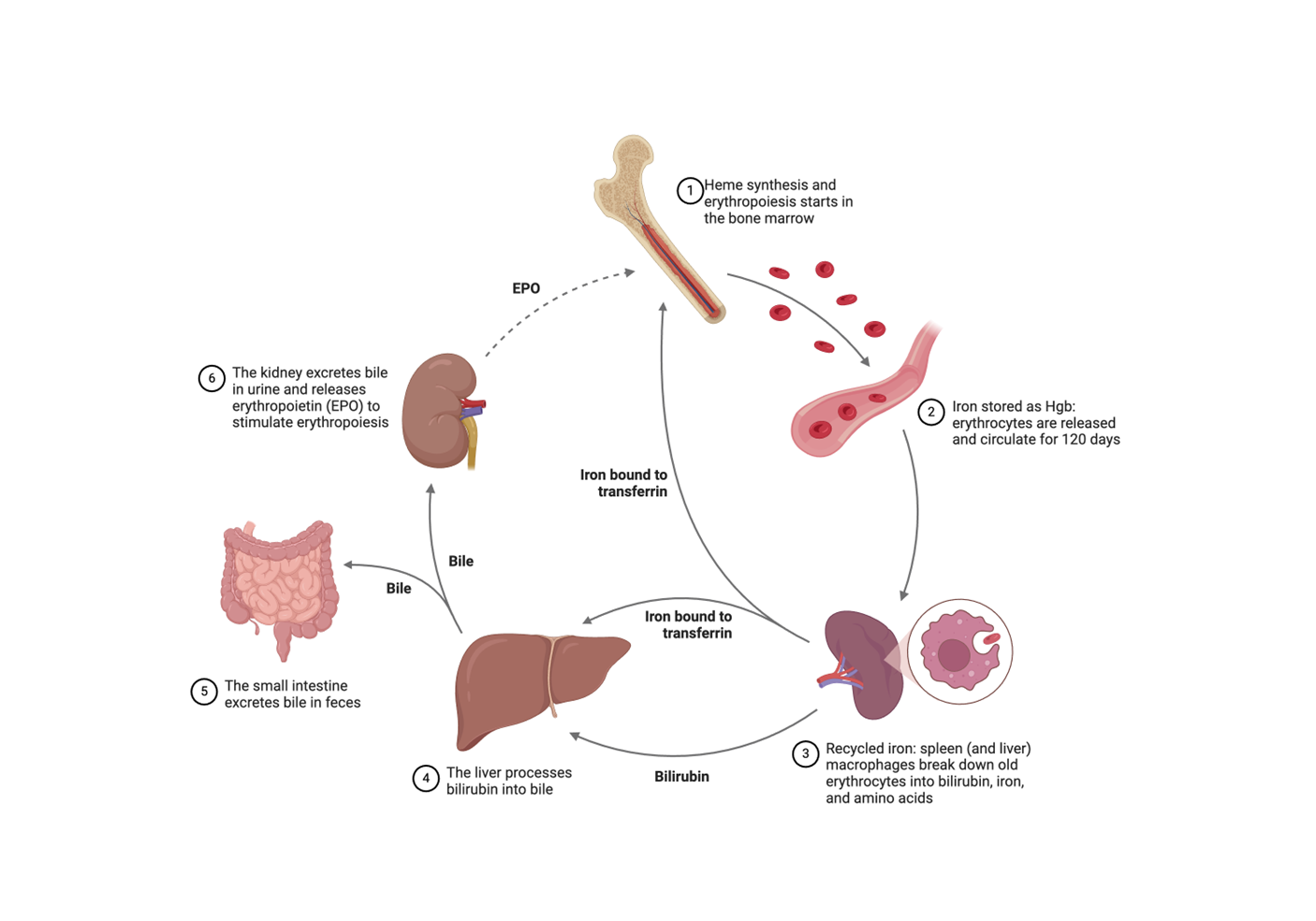
Iron homeostasis depends on:
Interaction of iron with proteins involved in iron absorption, transport, retention, export
Liver
Major storage site for iron storage
“Command central” of iron homeostasis
hepcidin
Master iron-regulating hormone
Controls the release of iron from enterocytes and macrophages into the circulation
Iron-Deficiency Anemia (IDA)- pathophysiology and clinical presentation
Insufficient iron for hemoglobin synthesis
•Koilonychia
•Dry mouth, glossitis
•Muscle dysfunction
•Inability to regulate body temp
•Pica
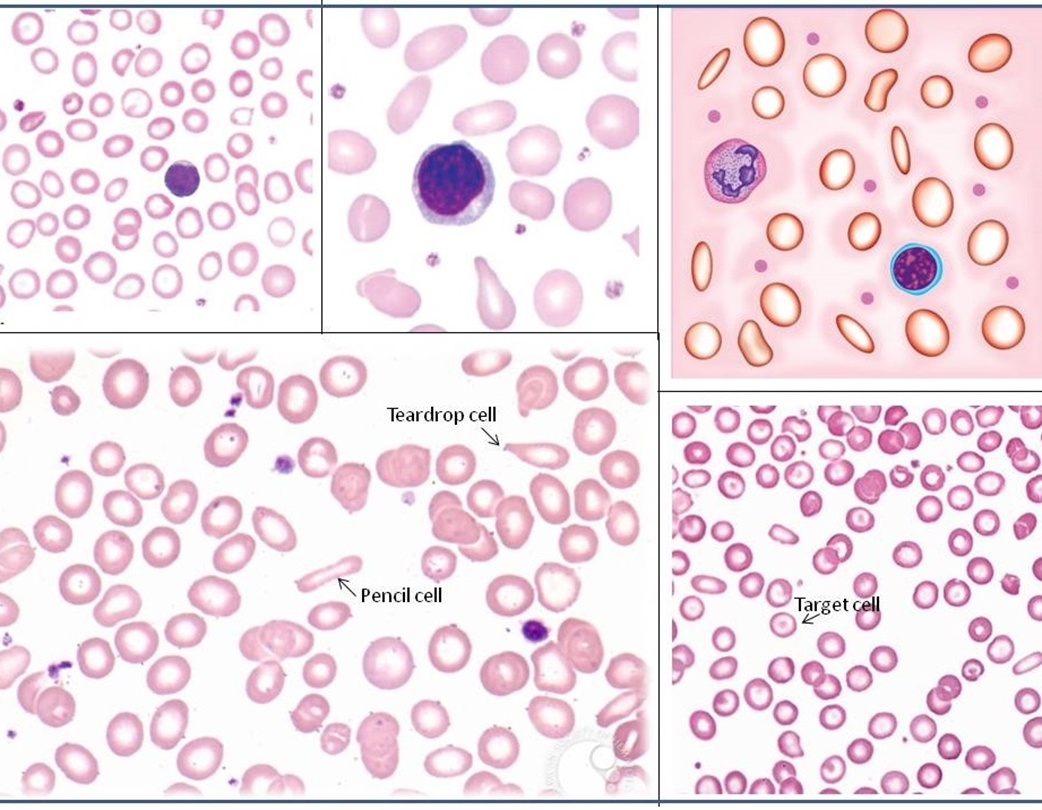
peripheral blood smear of IDA: size, color and morphology of cells
•MCV 55-74 fL – microcytic
•MCHC 22-31 g/dL – hypochromic
•RDW will be increased – anisocytosis
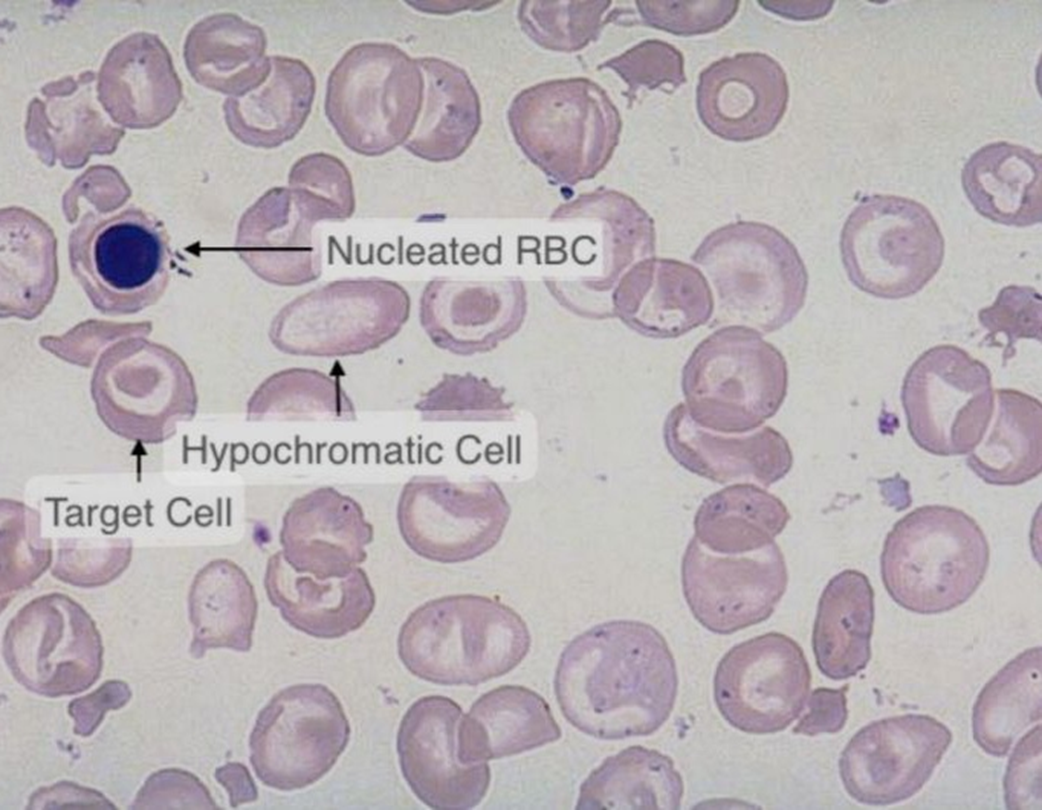
•Poikilocytes - •Target cells •Elliptocytes •Teardrop cells
reticulocyte count of IDA
low - not enough iron to make hb → less cells
iron studies of ida
Low serum iron <30 ug/dL
Low serum ferritin <12 ug/dL
High TIBC (total iron binding capacity - not enough iron = more room to bind)
iron deficiency anemia - look for color and size first - microcytic, hypochromic
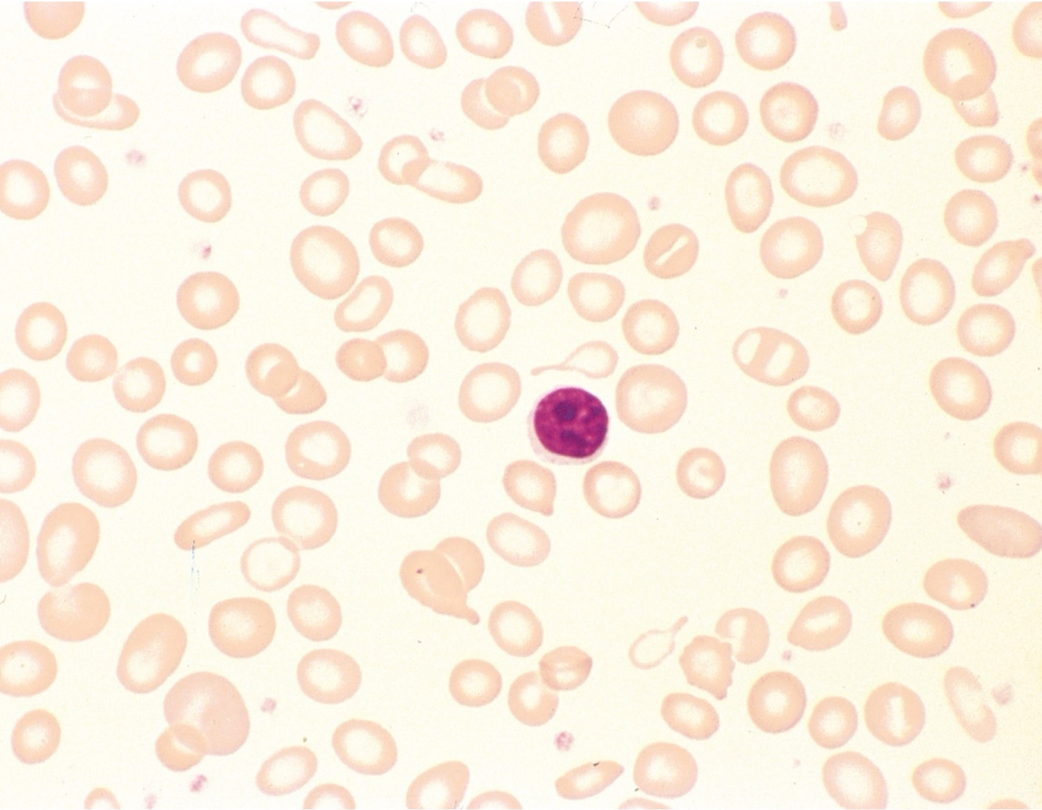
iron deficiency anemia
list causes of IDA
Blood loss
Lack of dietary iron (strict vegetarianism or poor diet)
Malabsorption
Defective iron utilization. Characterized by ineffective RBC formation and the presence of ferritin in developing RBCs. (Sideroblastic anemia)
Defective iron reutilization. This is the second most common form of anemia and is related to a variety of chronic diseases, infections, and cancers
Obesity
Thalassemia
genetic blood disorder caused by mutations in one or more globin genes (alpha or beta) responsible for hemoglobin production.
These mutations result in decreased or absent synthesis of globin chain(s)
different kinds of thalassemia
•> 400 unique mutations
•α-thalassemia
•β-thalassemia
thalassemia pathophysiology
The imbalance in globin chain production leads to ineffective erythropoiesis and hemolysis. The excess unpaired globin chains precipitate in red blood cell precursors, causing their premature destruction in the bone marrow (ineffective erythropoiesis) or spleen (hemolysis).
thalassemia Clinical Presentation:
Symptoms: Fatigue, weakness, pallor, and jaundice
Severe Cases: Growth retardation, bone deformities, and splenomegaly
Complications: Heart failure, liver disease, and endocrine dysfunction due to iron overload from frequent blood transfusions
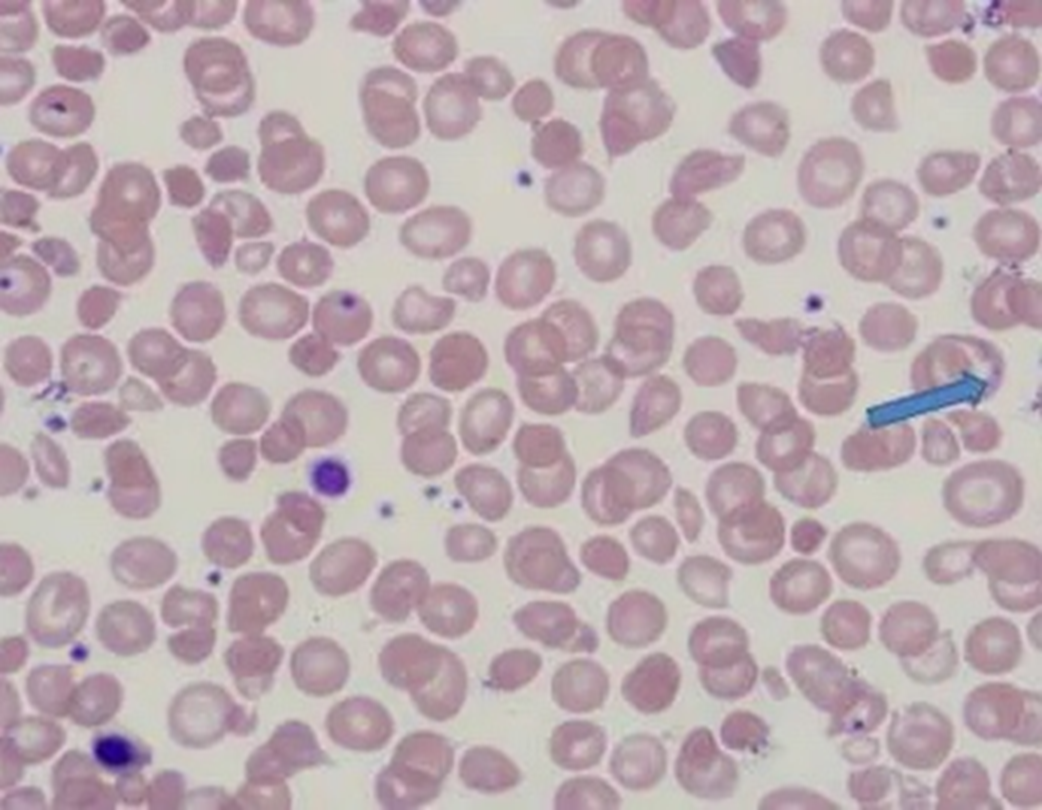
Peripheral blood smear of thalassemia: size, color, morphology, and inclusion bodies
•MCV 55-74 fL – microcytic
•MCHC 22-31 g/dL – hypochromic
•RDW will be increased
•Target cells, basophilic stippling - telltale sign, NRBCs
Reticulocyte count of thalassemia
high
Hgb electrophoresis of thalassemia
Abnormal hemoglobin patterns, reduced or absent specific globin chains.
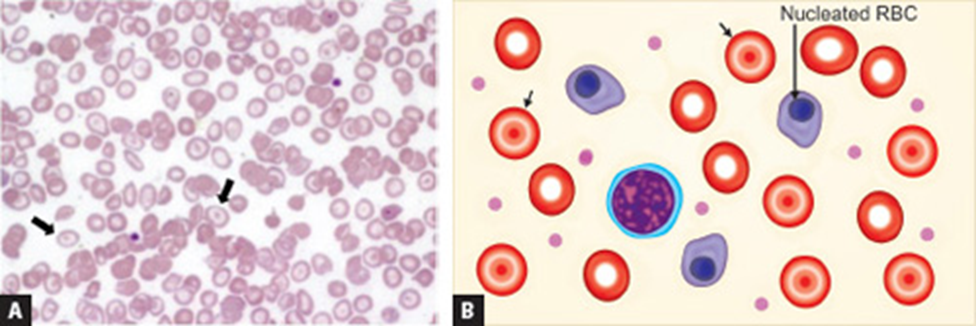
thalassemia
thalassemia
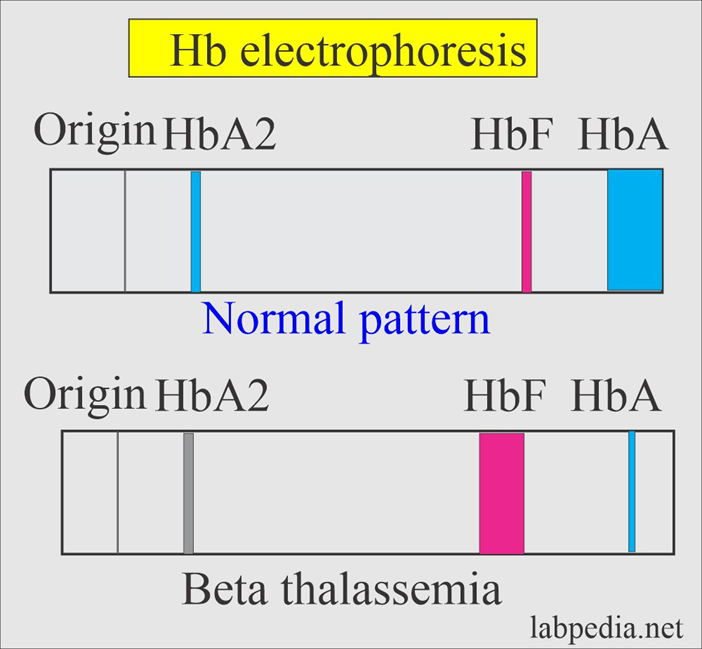
thalassemia, fetal hb is up, hbA decreases, hb origin decreased
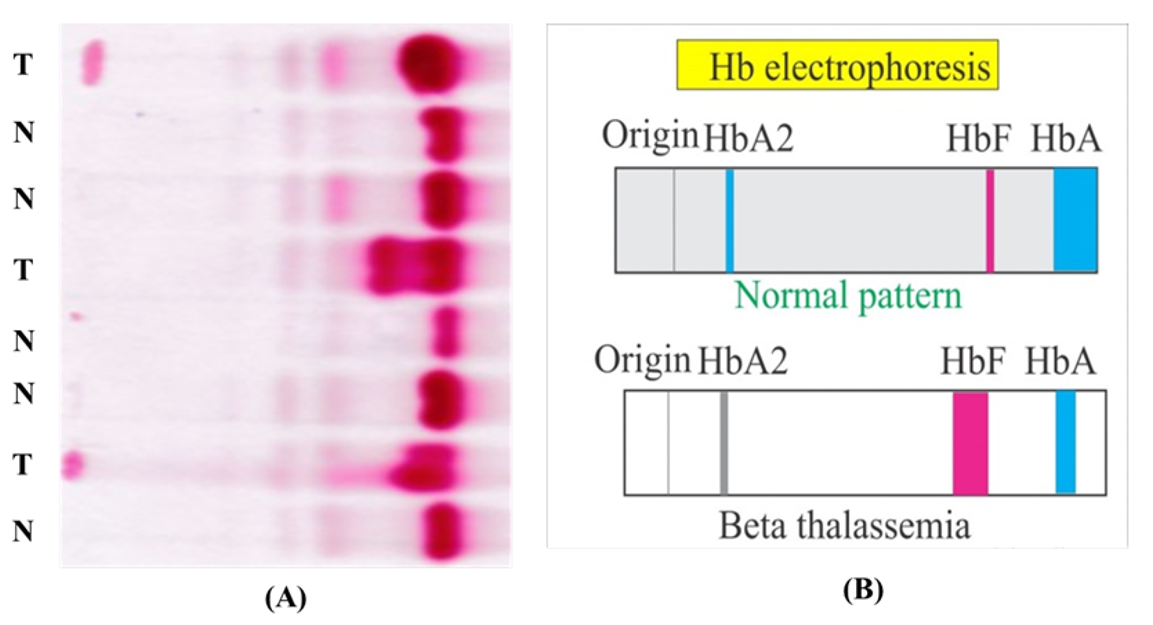
normal vs thallasemia hb electrophoresis
why do thalassemic patients have high levels of hbF?
To compensate, the body increases the production of gamma chains,
thalassemia
thalassemia
Anemia of Chronic Disease (acd) etiology
underlying chronic conditions such as infections, autoimmune diseases, chronic kidney disease, and malignancies lead to altered iron metabolism and impaired erythropoiesis.
Pathophysiology: ACD is characterized by
Increased hepcidin levels, which inhibit iron absorption and release from macrophages.
Shortened red blood cell lifespan due to increased phagocytosis.
Impaired response to erythropoietin, reducing red blood cell production.
Inflammatory cytokines, like IL-6, further suppressing erythropoiesis and iron availability.
How do cytokines/inflammation cause ACD?
In response to inflammatory signals, monocytes and T cells become activated and release cytokines
A. IL-6 increase hepcidin levels, which in turn bind and degrade ferroportin, trapping Fe3+ within the M𝞅 decrease, erythropoiesis.
B. IL-1 inhibits EPO release from kidneys, decreasing erythropoiesis.
C.TNF and IFN inhibit RBC progenitor proliferation
D.TNF increase erythrophagocytosis by M𝞅
clinical presentation of ACD
Mild to moderate anemia symptoms: fatigue, weakness, and pallor.
Symptoms of the underlying chronic condition (e.g., joint pain in rheumatoid arthritis, fever in chronic infections).
Complete Blood Count (CBC) of acd: cell size
Mild to moderate anemia, normocytic or microcytic
Serum Ferritin of acd lab eval
Normal or increased (reflecting adequate iron stores)
Serum Iron, Transferrin, and TIBC:
Low or decreased, tbic low because stores are filled with iron, so not being used
Bone marrow evaulation of acd
↑ M:E ratio
Poor hemoglobin production in erythroblasts
Sideroblasts < 30% - holding onto iron
Increased hemosiderin in macrophages - macrophages holding onto iron
Sideroblastic Anemias
anemias result from a mutation that affects the first enzymatic step of heme synthesis (formation of ALA).
heme synthesis accumulates in mitochondria, but in this anemia, you don’t get heme so iron deposits in these cells
Sideroblastic Anemias etiology
can be caused by a variety of factors, including genetic mutations (congenital sideroblastic anemia), acquired conditions (such as myelodysplastic syndromes), chronic alcoholism, lead poisoning, and certain medications (e.g., isoniazid, chloramphenicol).
Sideroblastic Anemias Pathophysiology
defective heme synthesis within the erythroid precursors in the bone marrow. This defect leads to
the accumulation of iron in the mitochondria surrounding the nucleus of the developing red blood cells → ring sideroblasts.
Disruption in normal RBC production
Sideroblastic Anemias clinical presentation
•Symptoms of anemia: fatigue, weakness, pallor, and shortness of breath.
•Hepatosplenomegaly (enlarged liver and spleen)
•Iron overload
•Symptoms related to hemosiderosis such as joint pain and diabetes mellitus
Complete Blood Count (CBC): sideroblastic anemia - color and size
•Normochromic and hypochromic
•Anisocytosis w/microcytes, macrocytes, and normocytes
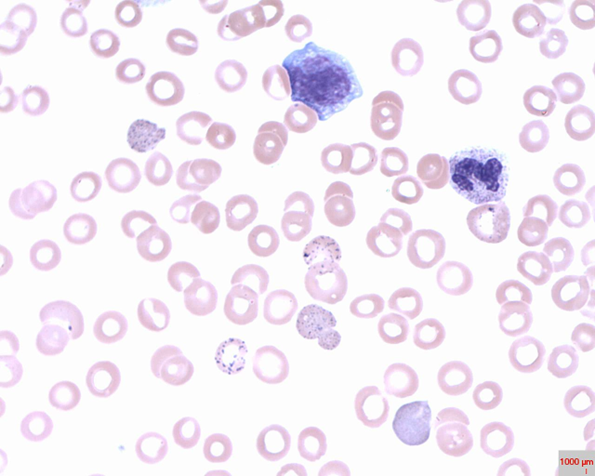
type of abnormal cell and inclusion body of sideroblastic anemia
•Target cells
•Inclusion bodies w/Pappenheimer bodies (iron deposits)
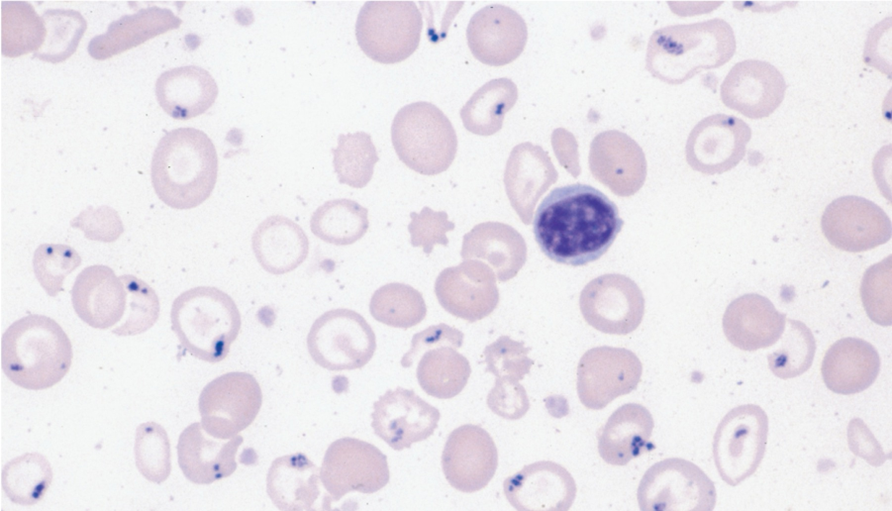
sideroblastic anemia
Bone Marrow Examination of Sideroblastic anemia
Ring sideroblasts > 40% of erythroblasts
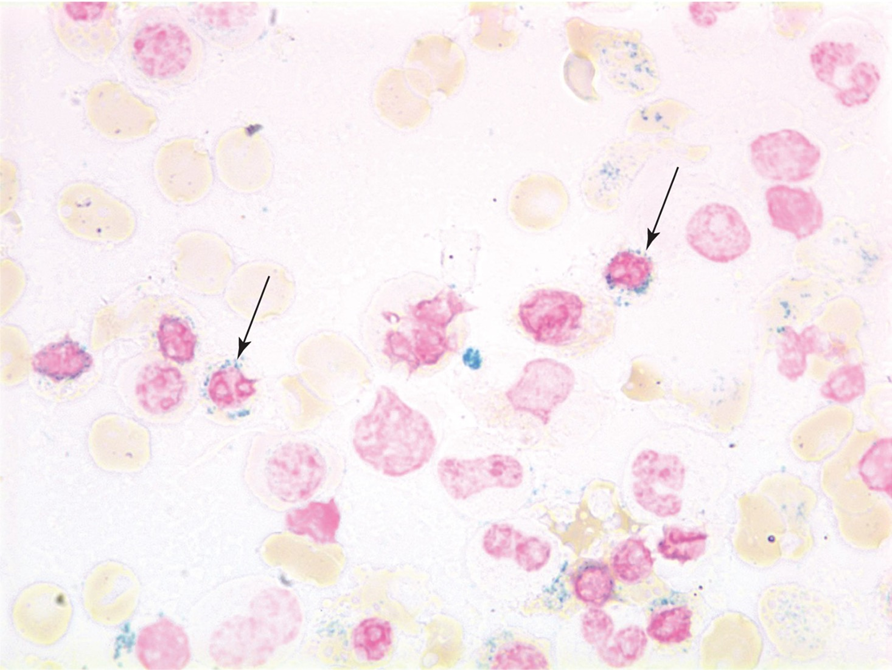
sideroblastic bone marrow