242 Lab 2: Ester Synthesis
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
what was this lab?
performing a fischer esterification by reacting an unknown alcohol with acetic acid under acidic conditions to get an ester
what is a limitation of the fischer esterification?
equilibrium usually favors carboxylic acid and ester evenly
how can we force the equilibrium towards ester?
1) one of the reactants can be used in excess, in this case acetic acid
2) can use a drying agent to remove product water which will drive the reaction forward
what analytical techniques were used?
IR (reactant and product) and NMR (product to see which starting alcohol)
safety information
glacial acetic acid and sulfuric acid are both corrosive (sulfuric acid especially nasty)
procedure in apparatus
assemble apparatus with 25 ml rb & west condensor, place unknown alcohol in rb, add acetic acid to rb, add 1 ml H2SO4, reflux 60 min & cool to room temp
procedure after cooled
disassemble apparatus and transfer rxn mixture to separatory funnel, wash sequentially with DI H2O, 5% aq NaHCO3, & sat. aq NaCl, transfer organic (ester) layer to flask & dry with Na2SO4, decant to get product liquid
mechanism for fischer esterification
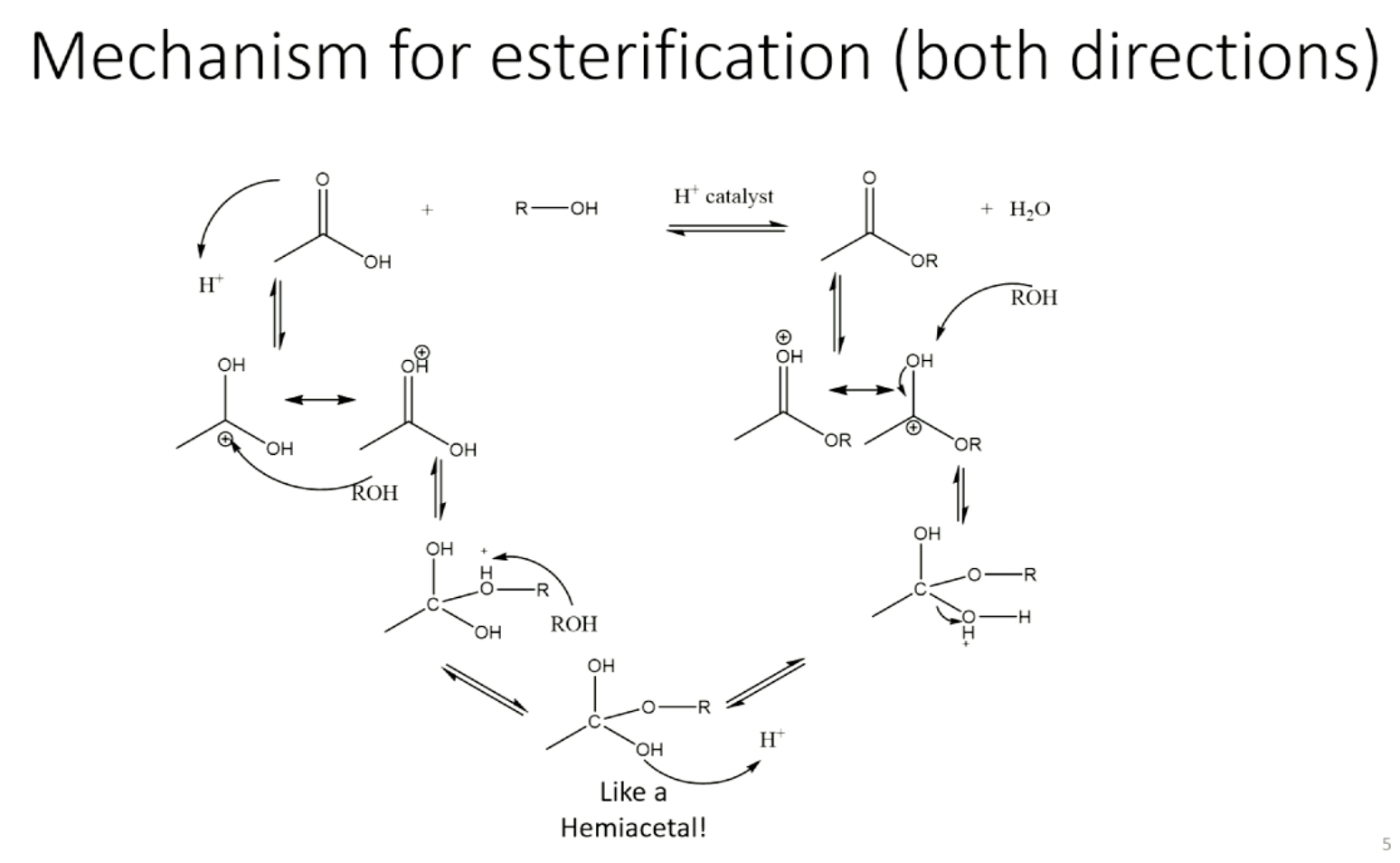

B) the alcohol is the limiting reagent so it’s important that all of the alcohol actually make it into the reaction flask

D) none of the above would change the ratio of ester to alcohol
what is transmittance in IR?
100 transmittance means all light is transmitted through sample, when some light is absorbed by molecule to cause molecule to vibrate, transmittance lowers because bond is activated
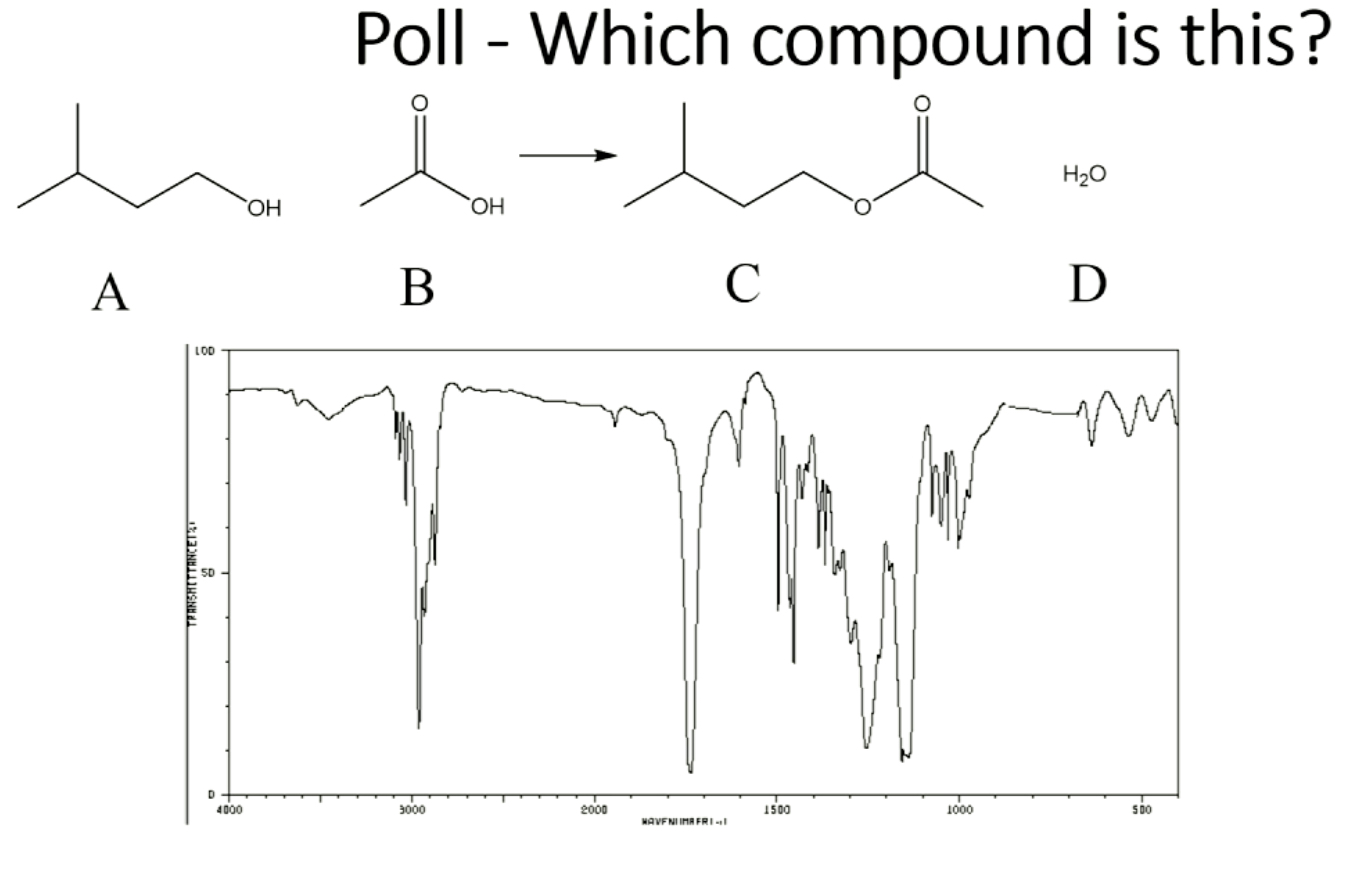
C) the little broad peak is noise (the size of the peak relates to polarity)
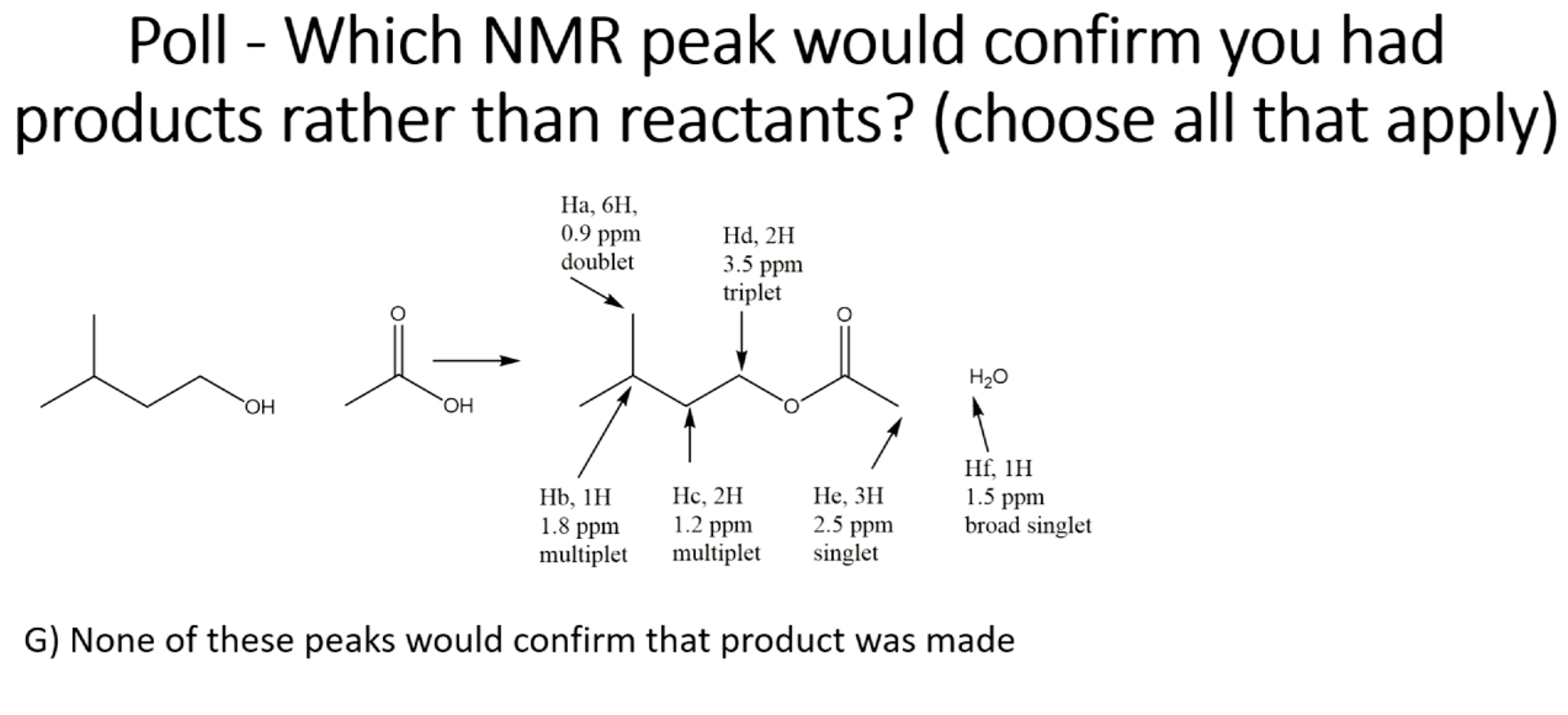
G) (Hf not because OH is unpredictable)
In this experiment you reflux your solvent using a water condenser. Why is that necessary rather then just heating an open flask?
refluxing allows the reactions to be kept as hot as possible without solvent evaporating away
if you spill concentrated sulfuric acid on your hand, what should you do first?
run your hand under lots of cold water and then get the TA’s attention
what are some of the dangers of concentrated sulfuric acid?
releases lot of heat when you add H2O to the acid which risks splattering, very reactive to many other reagents which risks side product formation, highly corrosive towards skin/clothing, causes severe damage if it ingested
______ is being used in excess in this lab in order to ______
acetic acid is being used in excess in this lab in order to push the equilibrium to favor more product
consider the transformation of pentanol into pentyl acetate. An argument in favor of pentanol having the higher bp/GC retention time is ______. An argument in favor of pentyl acetate having the higher bp/GC retention time is ________. Based purely on these argument_________.
it can do H-bonding, it has the higher MW, it’s not clear which compound will have the higher BP and the only way to know for sure is to measure it.
the GC-MS library might give the name “acetic acid, butyl ester”. what does this mean?
your compound is butyl acetate since that is a synonym
what compound(s) will be removed from your product at some point during the extractions?
sulfuric acid, acetic acid, water
with a gibbs free energy=0, assume reaction taking place at 100 C, what is the equilibrium constant?
Keq=1
assume we carry out ester rxn under 1M acetic acid & 1M butanol, based on Keq=1, what is % yield of ester?
50
set up ICE table (initial, change, equilibrium), Keq=products²/reactants², solve for x which will be the %
now assume we carry ester rxn under conditions used in lab, so acetic acid is solvent. conc of acetic acid is 17M, with Keq=1 & 1M butanol & 17M acetic acid, what is % yield of ester?
93
set up ICE table, Keq= products²/reactants², x=the % ester