pH scale
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
What is the self-ionisation of water + its equation?
It involves one water molecule donating a hydrogen ion to another hydrogen ion, forming hydronium + hydroxide ions
H2O + H+ + OH-
How do you use Kw to calculate concentration?
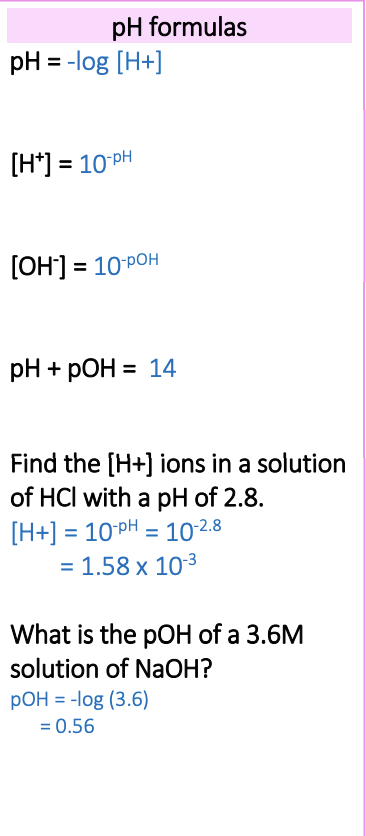
Use the variety of formulas, including; -log(H+), 10^-pH, 10^-pOH, pH + pOH = 14

What is the formula for pH + pOH?
pH + pOH = 14
What are the pH formula?
pH neutralisation reaction steps:
1) Determine no. of moles of acid and base
2) Calculate the moles of excess H+ and OH- according to the mole ratio in the balanced chemical equation
3) add volumes of acid + base to get a total volume
4) find (H+) or (OH-) using n/v
5) Calculate pH or pOH. if pH is found, use pH=14-pOH