Ch9: Atomic and Nuclear Phenomena
0.0(0)
0.0(0)
Card Sorting
1/45
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
46 Terms
1
New cards
Photoelectric Effect
e- ejection from metal surface from high frequency light
2
New cards
Current
Net charge flow/time from e-
Increase light intensity = Increase current
Increase light intensity = Increase current
3
New cards
Threshold Frequency (fT)
Min light frequency causing e- ejection
Depend on chemical composition of material/metal
Depend on chemical composition of material/metal
4
New cards
f < fT
No e- ejected
Not enough photon energy
Not enough photon energy
5
New cards
f > fT
e- ejected
Max Ek = hf - hfT (work function)
Max Ek = hf - hfT (work function)
6
New cards
Photons
Light quanta
7
New cards
Frequency vs Wavelength
Increase frequency = Decrease wavelength = Increase photon energy
8
New cards
Electron Kinetic Energy
Excess energy above Ft converted to e- kinetic energy
Increase light energy = Increase atom electrical potential energy = Increase e- kinetic energy
Increase light energy = Increase atom electrical potential energy = Increase e- kinetic energy
9
New cards
Maximum Electron Kinetic Energy
All photon energy transferred to e-
10
New cards
Work Function (W)
Min energy to eject e-
Depend on chemical composition of material/metal
Depend on chemical composition of material/metal
11
New cards
Particle Theory of Light
Light contains discrete energy bundles not a continuous wave
Supported by photoelectric effect
Supported by photoelectric effect
12
New cards
Photoelectric Effect: Change Light Colour
Change speed of e- ejected
13
New cards
Photoelectric Effect: Change Light Intensity
Change number of e- ejected
14
New cards
Bohr Model
Stable and discrete e- levels (orbits)
15
New cards
Bohr Model: Photon Absorption
e- jump from low-energy to high-energy orbit
Same frequency as energy diff between orbits
Same frequency as energy diff between orbits
16
New cards
Bohr Model: Photon Emission
e- fall from high-energy to low-energy orbit
Same frequency as energy diff between orbits
Same frequency as energy diff between orbits
17
New cards
Absorption Spectra
Impacted by small molecular structure changes
18
New cards
Infrared (IR) Spectroscopy
Determine chemical structure of compounds
Diff bonds absorb diff light wavelengths
Diff bonds absorb diff light wavelengths
19
New cards
UV-Vis Spectroscopy
Determine visible and UV light absorption
20
New cards
Indicators
Diff absorption patterns based on protonation state
Usually have conjugated double bonds or aromatic rings
Usually have conjugated double bonds or aromatic rings
21
New cards
Fluorescence
Exciting fluorescent substance with UV radiation
Excited e- return to ground state in 2+ steps emitting photons in process
Photon energy > Fluorescence radiation
Excited e- return to ground state in 2+ steps emitting photons in process
Photon energy > Fluorescence radiation
22
New cards
Mass Defect
Nuclei mass smaller than protons + neutrons
Unbonded nucleon mass - bonded nucleon mass
Mass converted to energy in nuclear fusion
Unbonded nucleon mass - bonded nucleon mass
Mass converted to energy in nuclear fusion
23
New cards
Nucleons
Protons and neutrons
24
New cards
Strong Nuclear Force
Attraction between protons and neutrons to form nucleus
Strongest force
Act over small distances
Strongest force
Act over small distances
25
New cards
Binding Energy
Diff in energy between bonded system and unbonded constituents energy levels
Radiated away (heat, light, EM radiation)
Radiated away (heat, light, EM radiation)
26
New cards
Nucleus Stability: Binding Energy
Iron: Stable nucleus, peak binding energy
Intermediate-sized nuclei: More stable than large or small
Intermediate-sized nuclei: More stable than large or small
27
New cards
Weak Nuclear Force
Small contribution to nucleus stability
28
New cards
4 Fundamental Forces of Nature
Strong nuclear force
Weak nuclear force
Electrostatic forces
Gravitation
Weak nuclear force
Electrostatic forces
Gravitation
29
New cards
Isotopic Notation
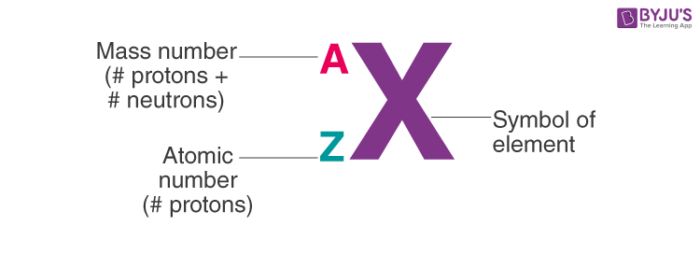
30
New cards
Fusion
Small nuclei combine into larger nucleus
Release energy
Release energy
31
New cards
Fission
Large nucleus split into smaller nuclei
Rarely spontaneous
Release energy
Rarely spontaneous
Release energy
32
New cards
Induced Fission
Fission reactions releasing more neutrons cause chain reaction of fission in nearby atoms
33
New cards
Radioactive Decay
Natural spontaneous nuclei decay emitting particles
34
New cards
Nucleon Conservation
Balanced nuclear decay reactions
Atomic number sum and mass number sum same on both sides
Atomic number sum and mass number sum same on both sides
35
New cards
Alpha Decay
Emit a-particle
Daughter nucleus A’ = A-4 and Z’ = Z-2
Daughter nucleus A’ = A-4 and Z’ = Z-2
36
New cards
a-Particle
4/2 He nucleus
2 protons, 2 neutrons, 0 electrons
Charge: +2
Very large
Non-penetrating
2 protons, 2 neutrons, 0 electrons
Charge: +2
Very large
Non-penetrating
37
New cards
Beta-Negative Decay
Emit b(-) particle and antineutrino
Daughter nucleus A’ = A and Z’ = Z+1
Daughter nucleus A’ = A and Z’ = Z+1
38
New cards
b-Particle
e-
Charge: -1
Very small
More penetrating
Charge: -1
Very small
More penetrating
39
New cards
Beta-Positive Decay (Positron Emission)
Emit positron (b+) and neutrino
Daughter nucleus A’ = A and Z’ = Z-1
Daughter nucleus A’ = A and Z’ = Z-1
40
New cards
Positron (e+)
e- mass with + charge
41
New cards
Gamma Decay
Emit y-rays
Detect on atomic absorption spectrum
Daughter nucleus A’ = A and Z’ = Z
Detect on atomic absorption spectrum
Daughter nucleus A’ = A and Z’ = Z
42
New cards
y-Rays
High energy/frequency photons
No charge
Lower parent nucleus energy
No charge
Lower parent nucleus energy
43
New cards
Electron Capture
Absorb inner e- to combine with proton
Form neutron and release neutrino
Daughter nucleus A’ = A and Z’ = Z-1
Form neutron and release neutrino
Daughter nucleus A’ = A and Z’ = Z-1
44
New cards
Half-Life (T 1/2)
Time for half of sample to decay
Remaining amount → 0
Remaining amount → 0
45
New cards
Half-Life Common Q: Given half-life and time passed
time passed/half-life = half-lives passed
(1/2)^half-lives passed = amount remaining
(1/2)^half-lives passed = amount remaining
46
New cards
Exponential Decay
Rate of radioactive nuclei decay proportional to number of nuclei remaining