Organic Chemistry - Nomenclature
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
44 Terms
Alkanes
-chain of carbons connected by single bonds
-ane
-CnH2n+2
Meth-
one
Eth-
two
Prop-
three
But-
four
Pent-
five
hex-
six
hept-
seven
oct-
eight
dec-
ten
undec-
eleven
dodec-
twelve
Naming alkanes
1. Find longest chain in the compound
2. Number the chain
3. Name the substituents
4. Assign a number to each substituent
5. Complete the name
Cycloalkanes
-CnH2n
-carbon atoms forming a ring
-Numbered starting at the point of greatest substitution
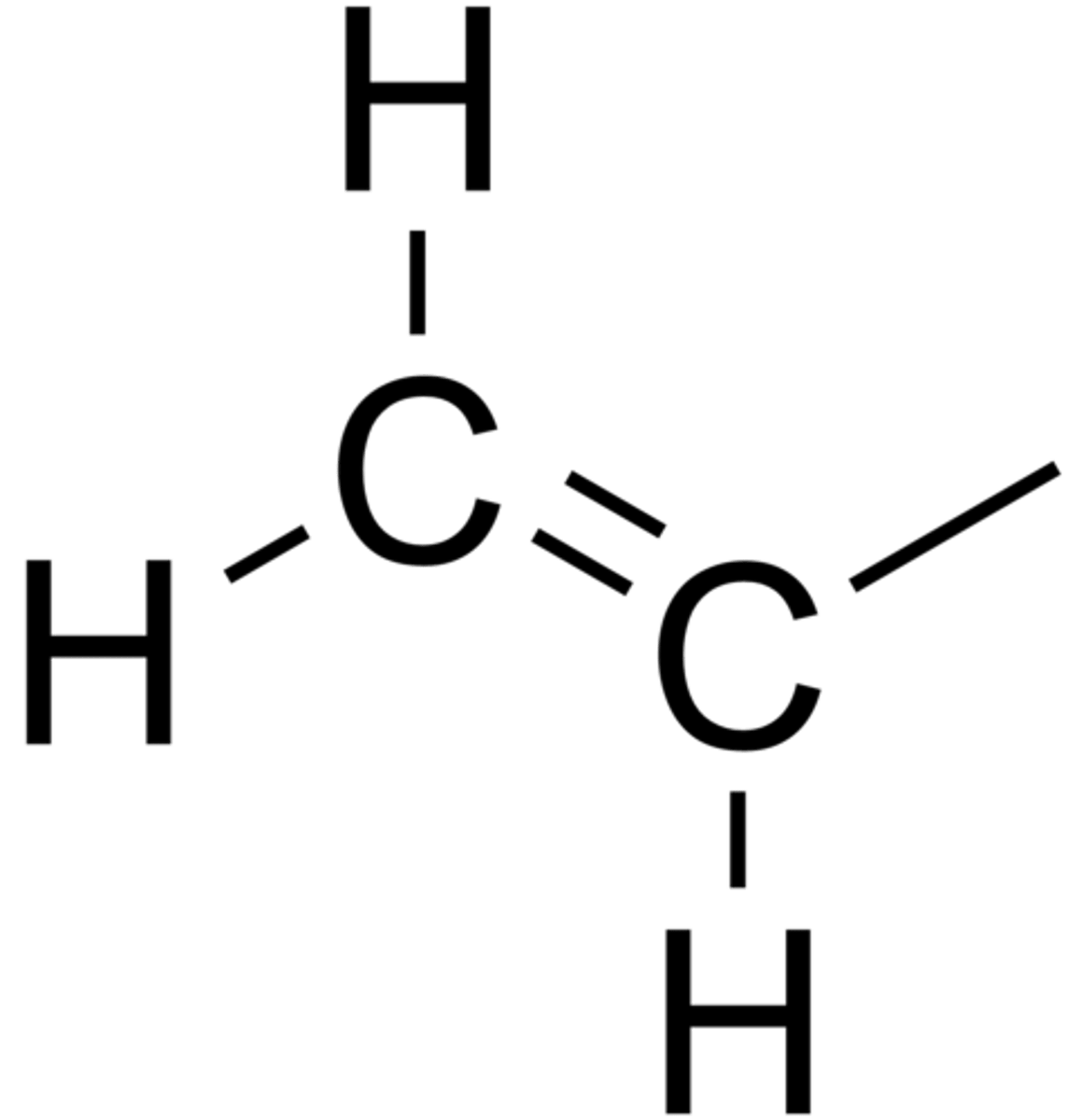
Alkenes
-olefins
-contain carbon-carbon double bonds
Vinyl
alkynes
carbon-carbon triple bonds
acetylene
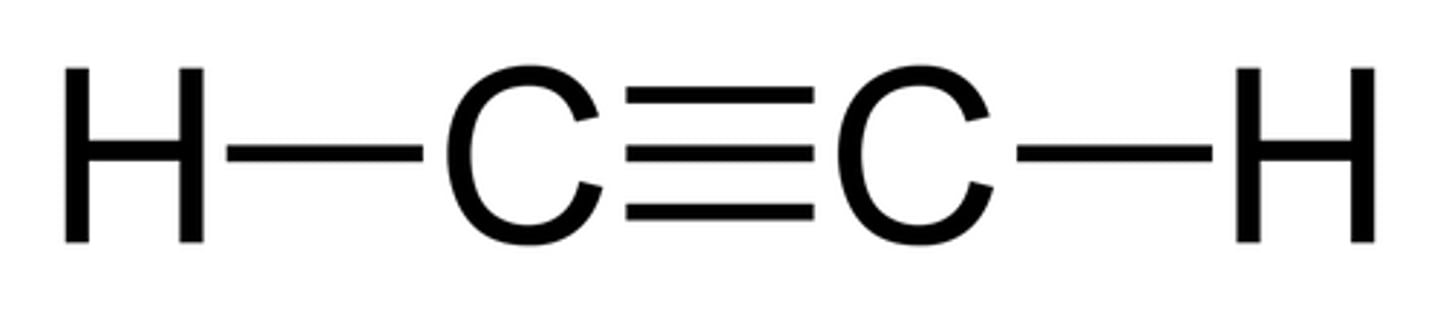
Haloalkanes
-contains halogen
-F, Cl, Br, I
-can also be named as alkyl halides
ex: ethyl chloride
alcohol
-replace "-e" of corresponding alkane with -ol.
-OH
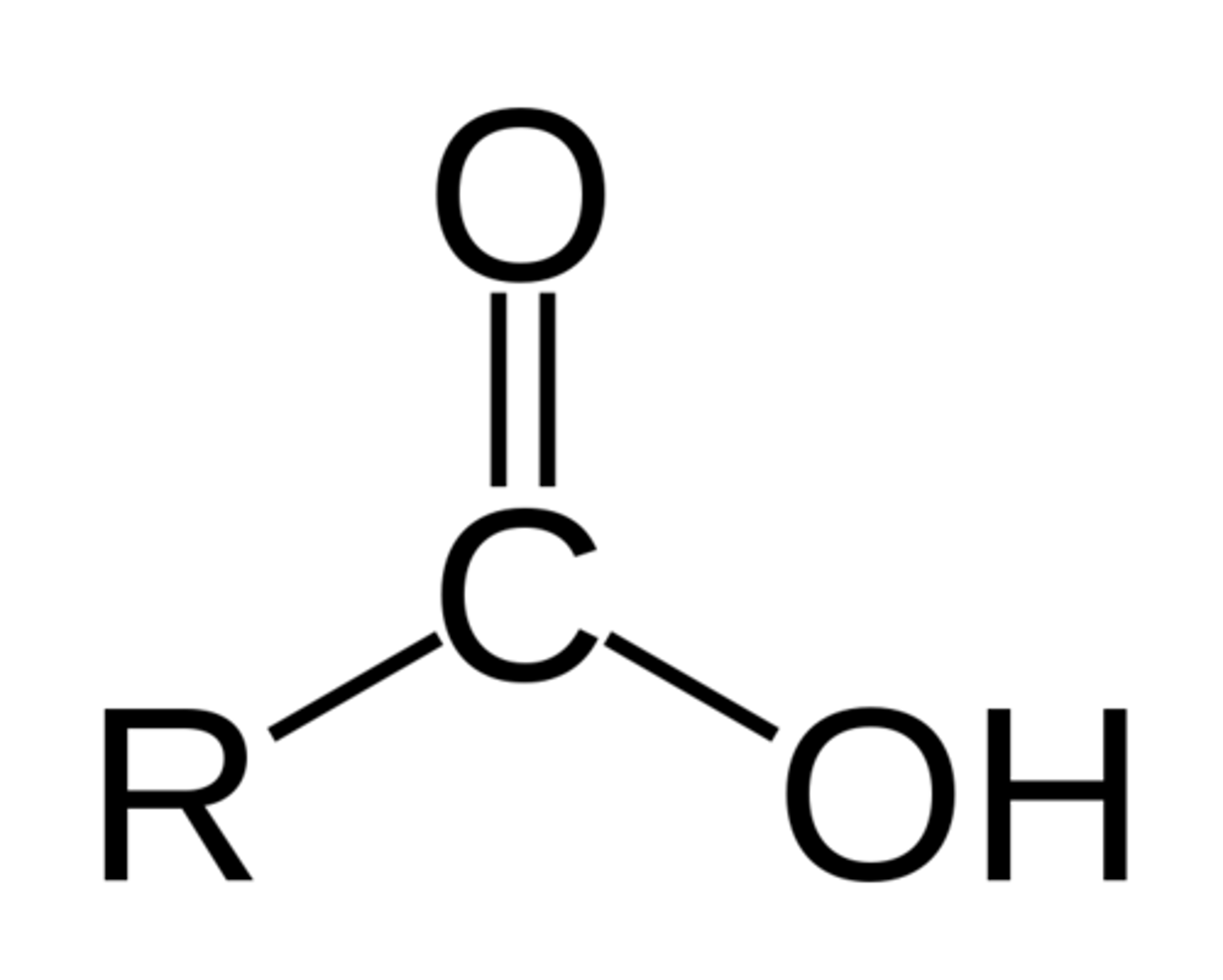
Carboxylic Acid
Pre: carboxy-
Suff: -oic acid
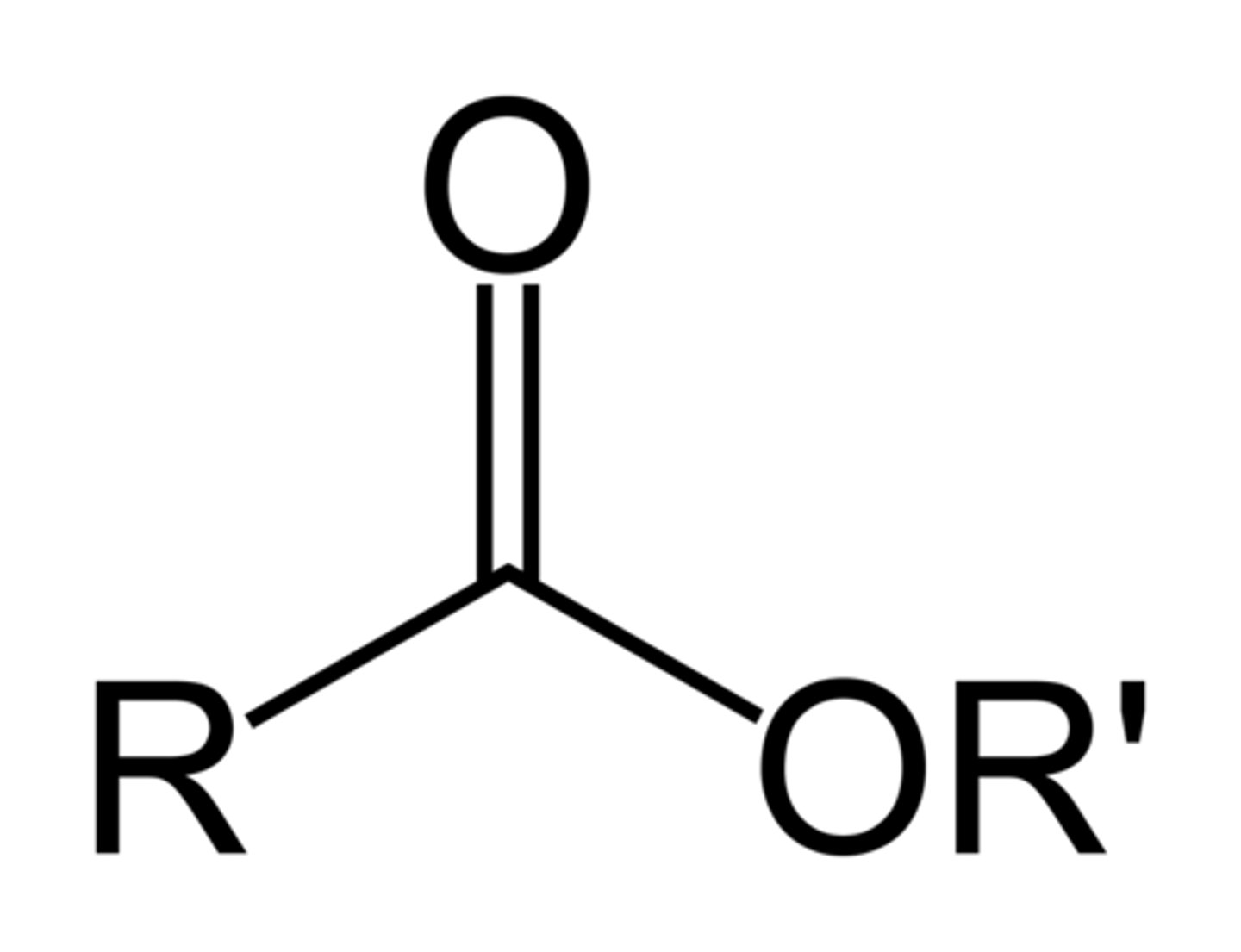
Ester
Pre: alkoxycarbonyl-
Suff: -oate
Acyl Halide
Pre: halocarbonyl-
Suff: -oyl halide
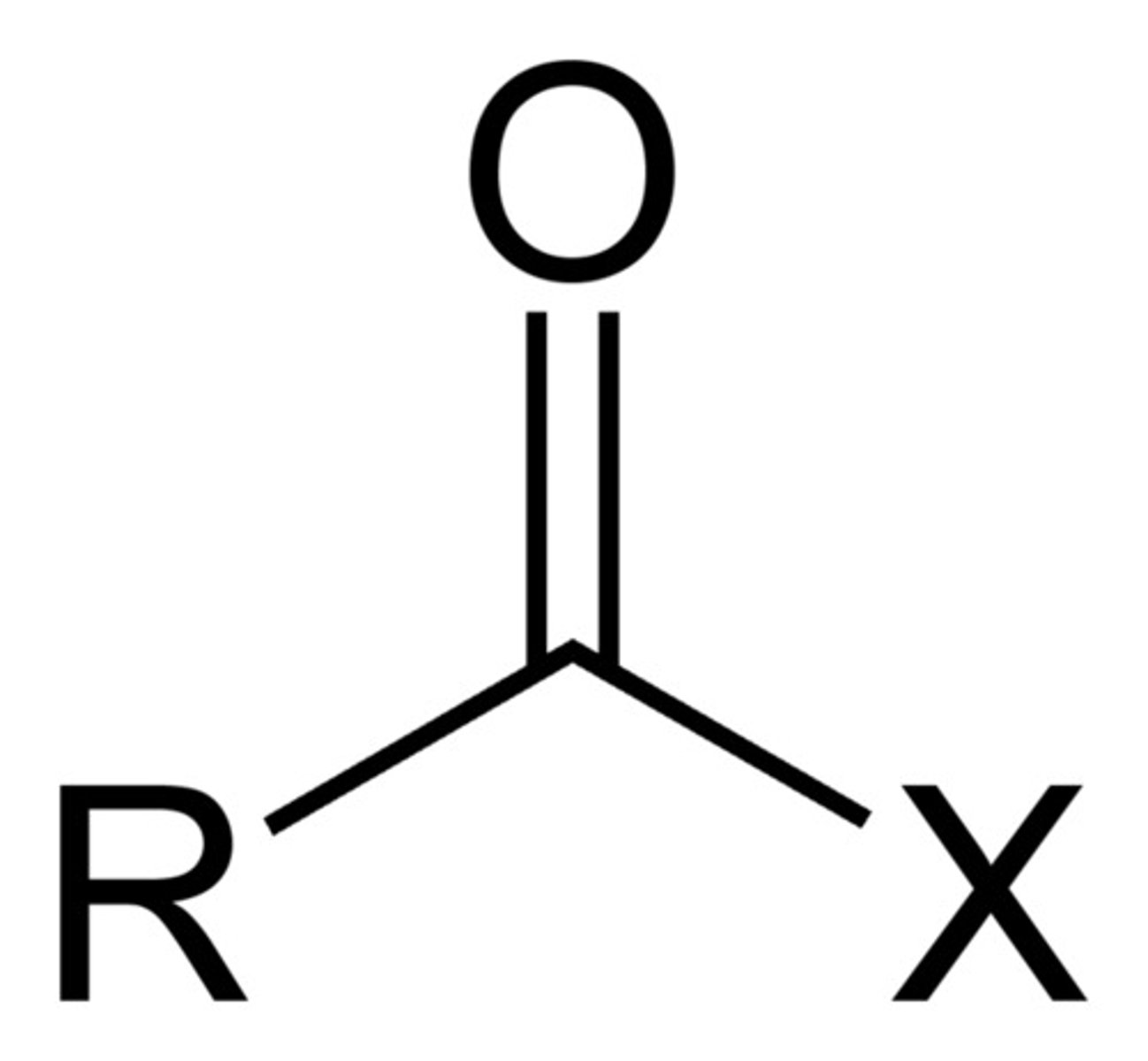
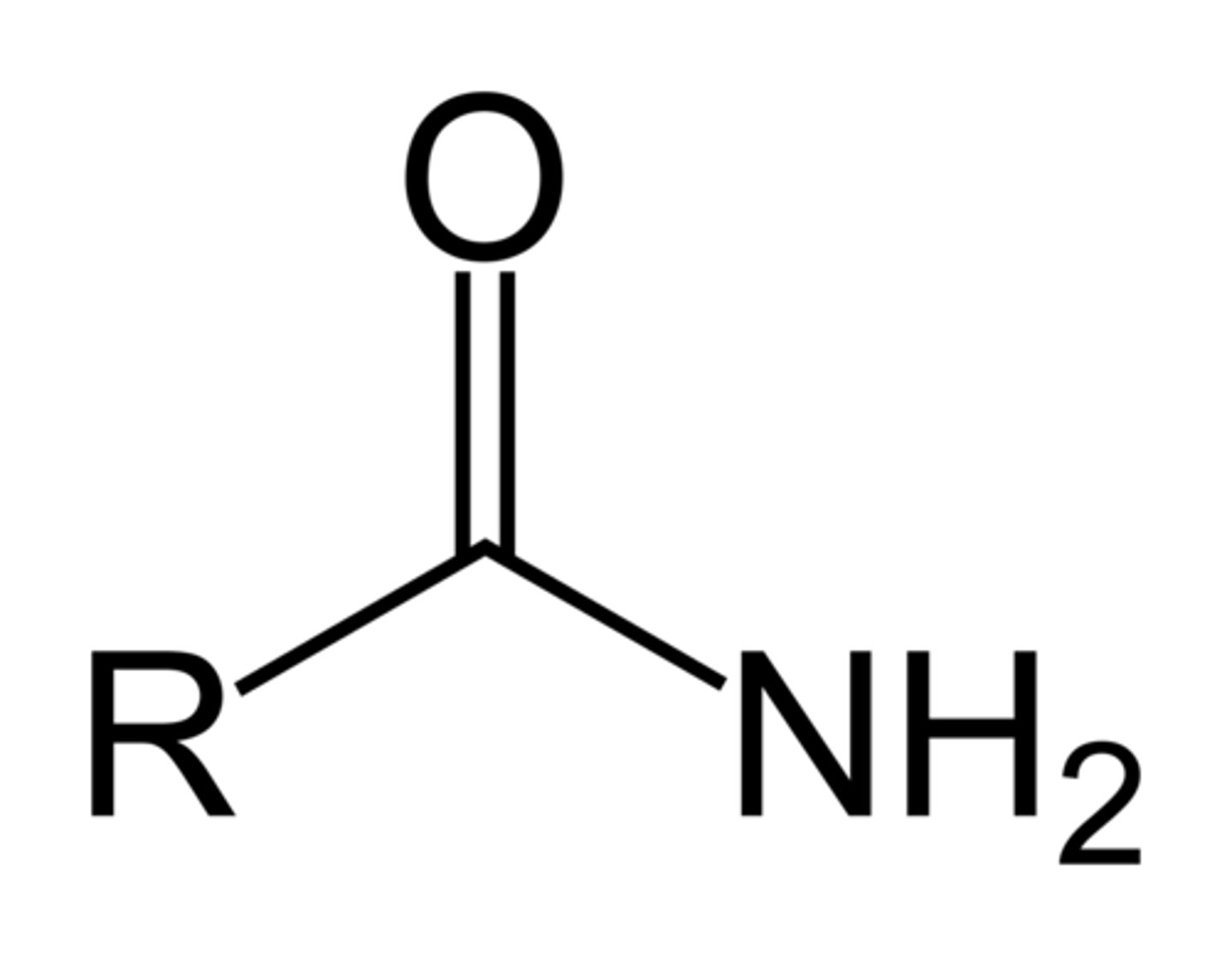
Amide
Pre: amido-
Suff: -amide
Nitrile/Cyanide
Pre: cyano-
Suff: -nitrile
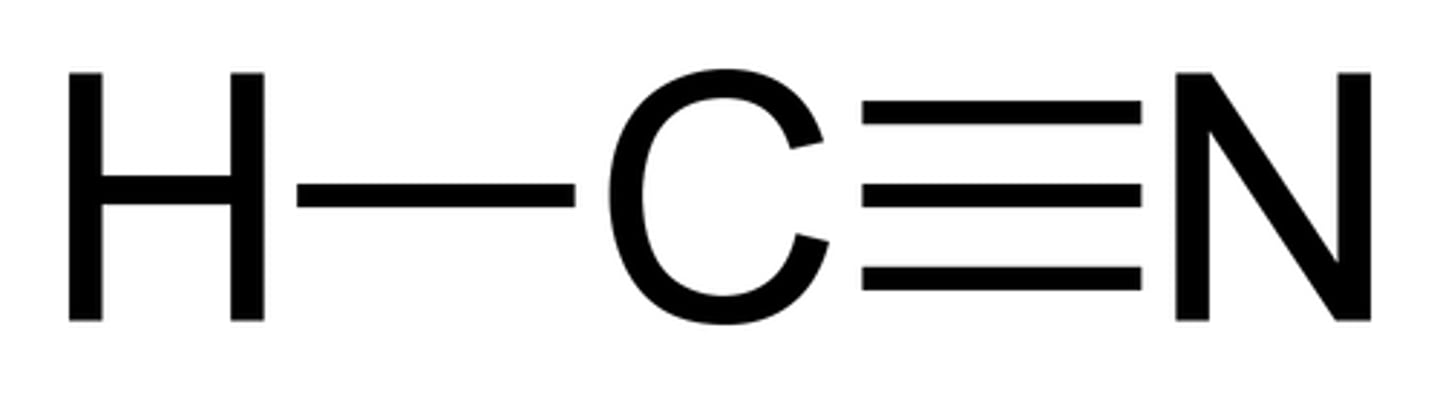
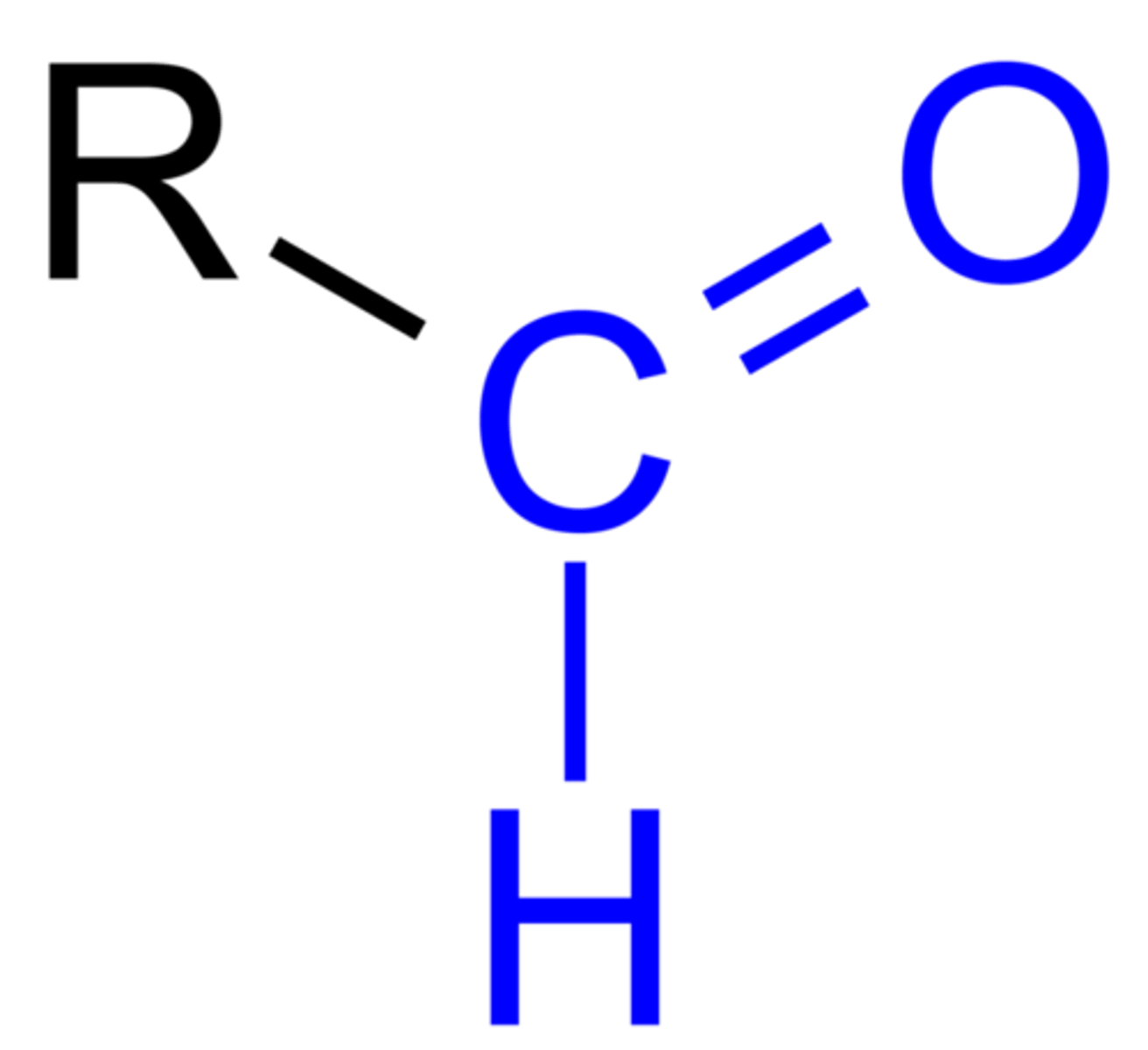
Aldehyde
Pre: oxo-
Suff: -al
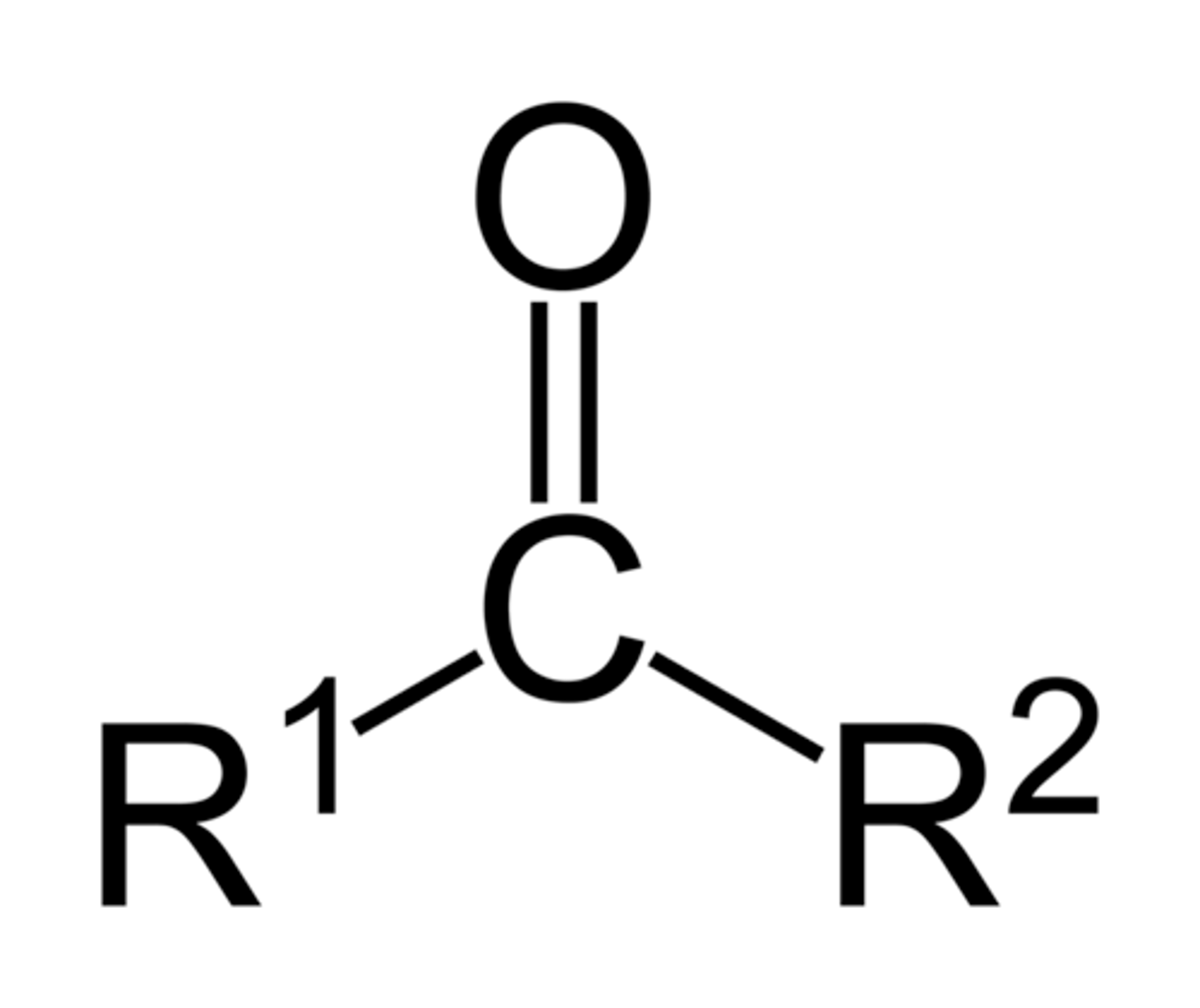
Ketone
Pre: oxo-
Suff: -one
Thiol
RSH
Pre: sulfhydryl-
Suff: -thiol
Amine
RNH2
Pre: amino-
Suff: -amine
Imine
R2C=NR'
Pre: imino-
Suff: -imine
Ether
ROR
Pre: -alkoxy
Suff: -ether
Sulfide
R2S
Pre: alkylthio-
Halide
-I, -Br, -Cl, -F
Pre: halo-
Nitro
RNO2
Pre: nitro-
Azide
RN3
Pre: azido-
Diazo
RN2
Pre: diazo-
tert
t-
*Use dash!
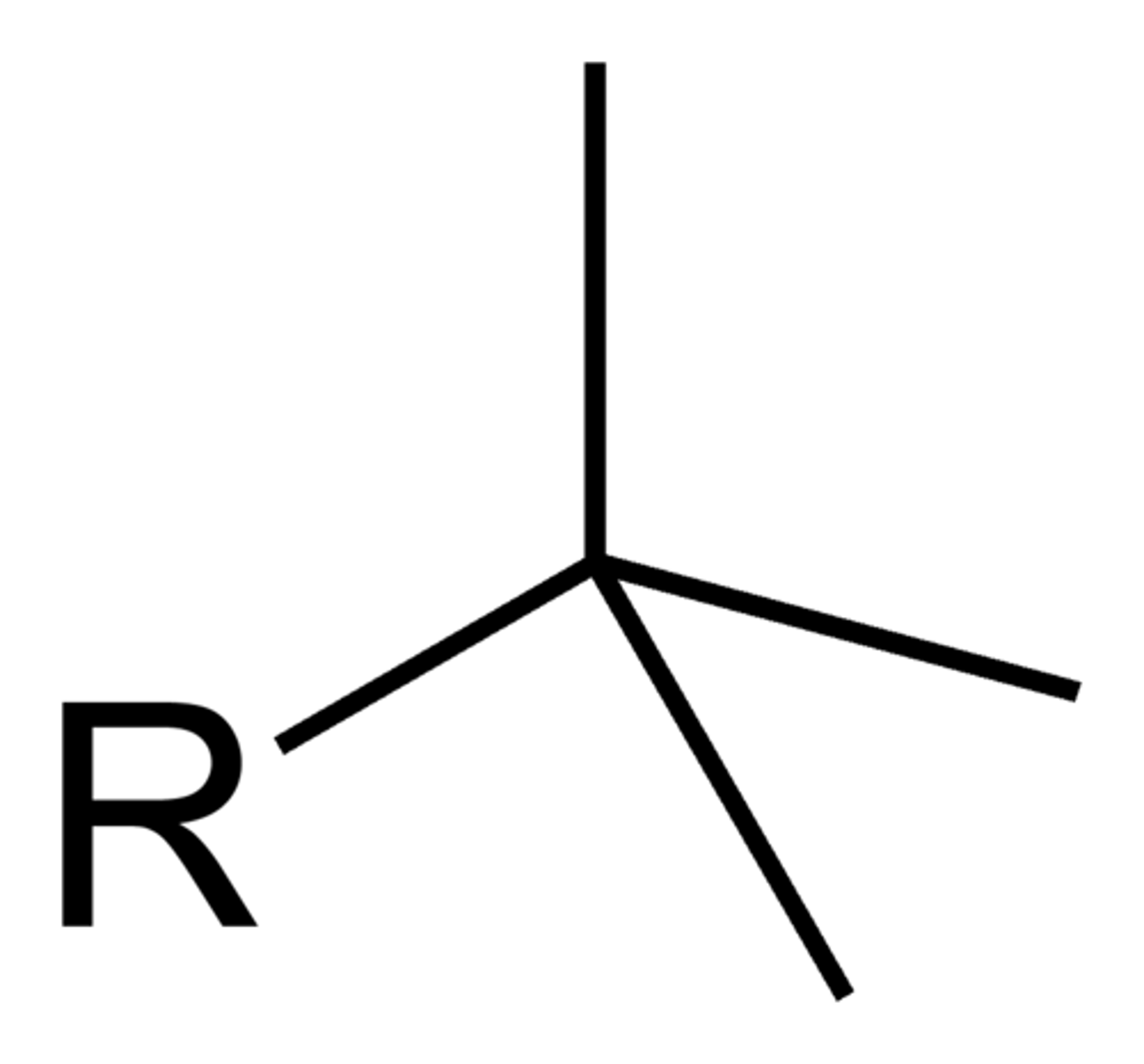
neo
-no dash!
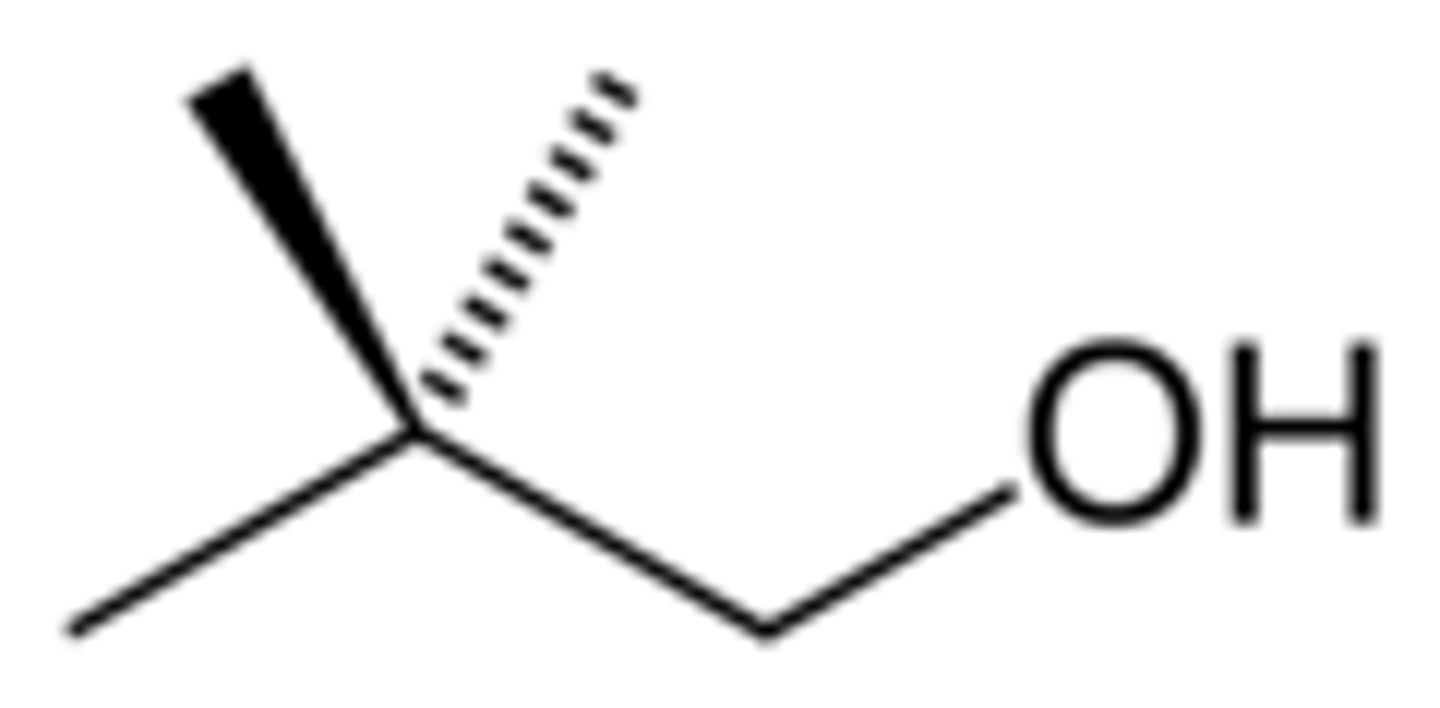
iso
-no dash!
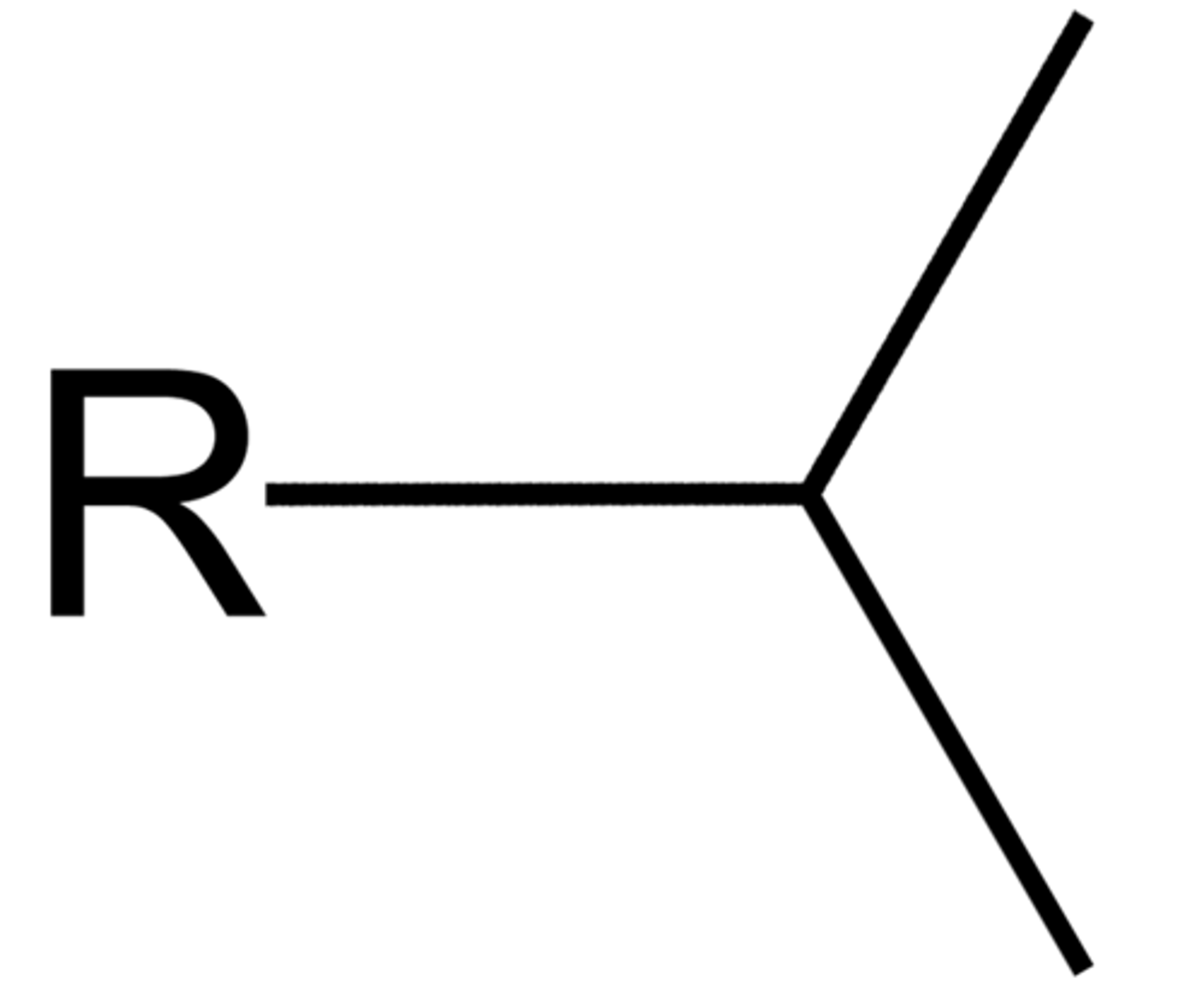
sec
-use dash! (sec-)
diols
-aka glycols
-Suff: -diol
-two numbers necessary to distinguish the two functional groups
vicinal
Diols with hydroxyl groups of adjacent carbons
geminal
diols with hydroxyl groups on the same carbon
-aka hydrates
oxiranes
three membered rings
-common name = epoxides