Chapter 3 Matter and Change (McGraw Hill Chemistry)
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
states of matter
includes liquids, solids, and gases. All existing matter is one of these. Can be classified by how it fills a glass.
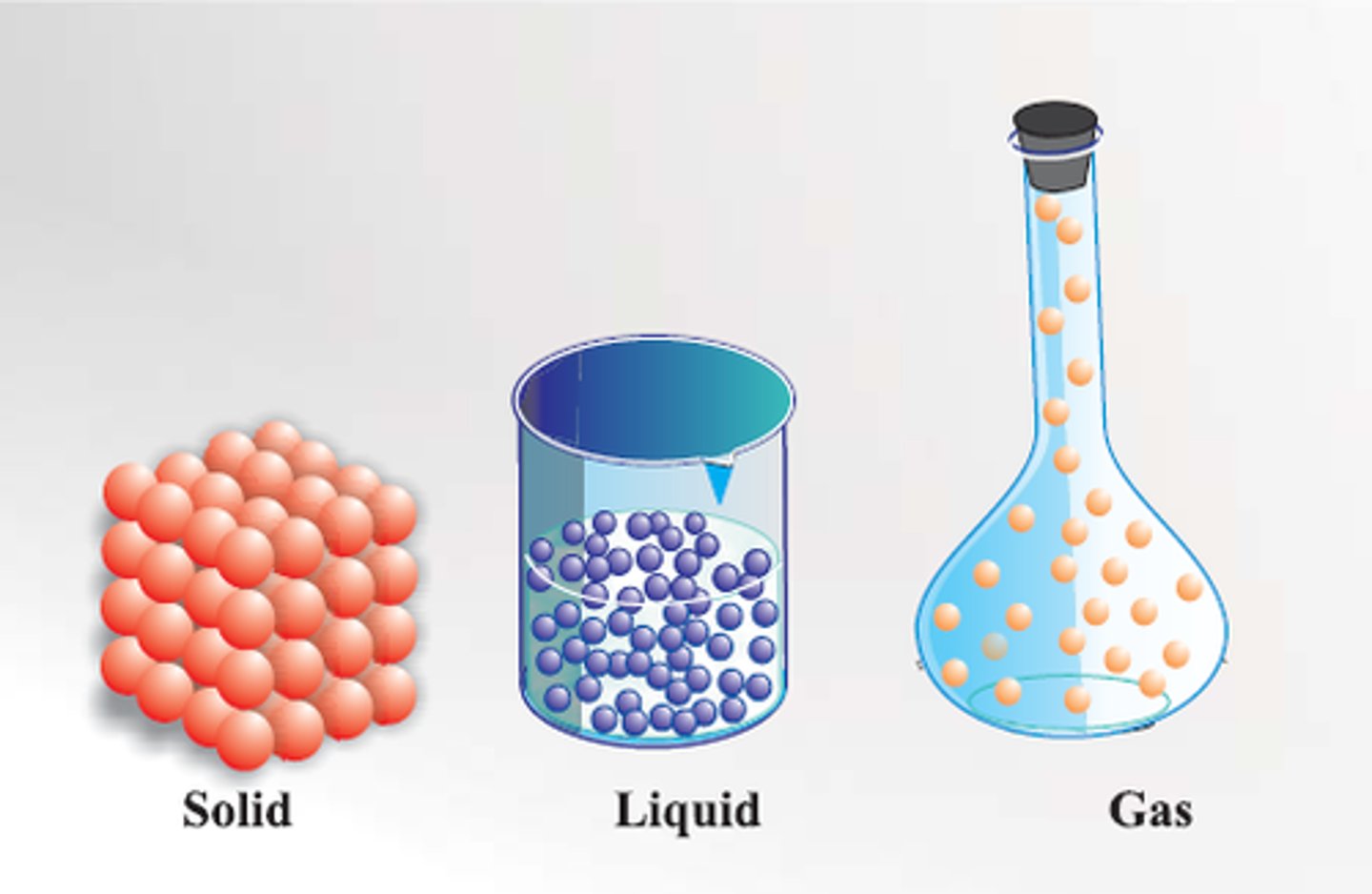
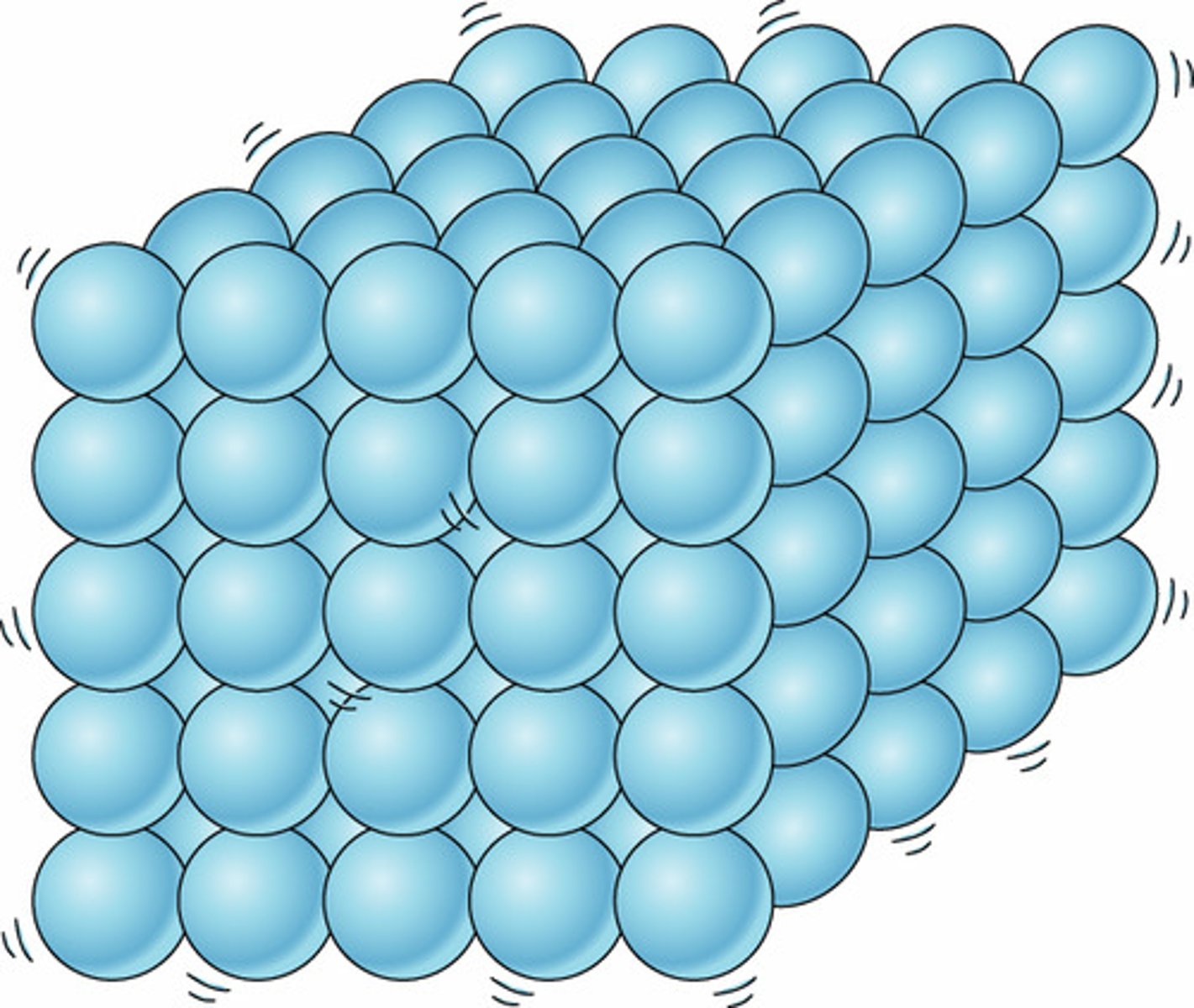
solid
has its own definite shape and volume. tightly packed particles. might not conform to a glass's shape.
liquid
state of matter that flows, takes the shape of its container. particles aren't rigid or tightly packed.
gas
flows to fit the gas, and fills up the glass.
vapor
gaseous state of a substance that is solid or liquid at room temperature.

physical property
characteristic of matter that can be observed without changing the sample's composition

extensive property
dependent of the amount of substance present
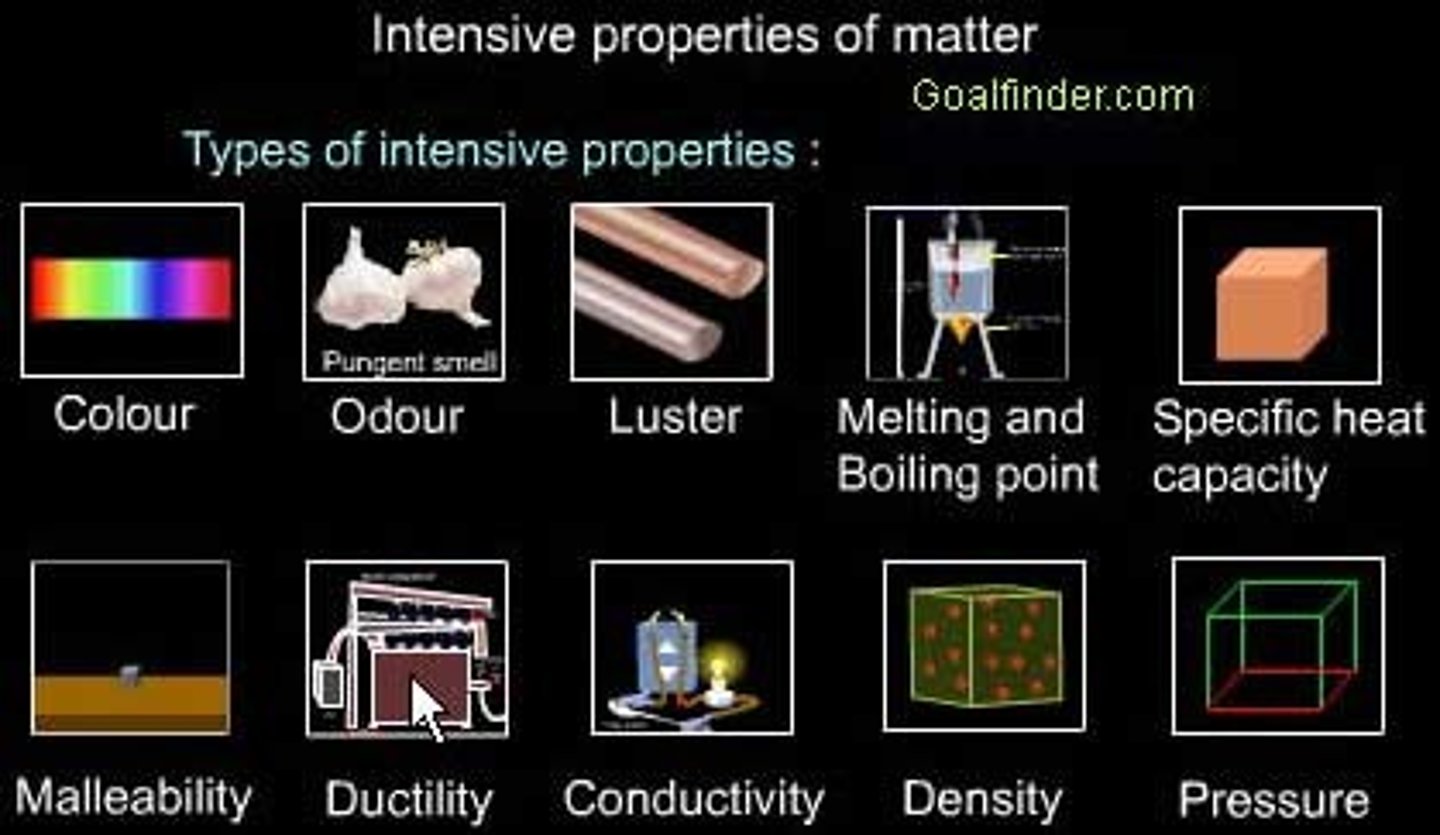
intensive property
independent of the amount of the substance present
chemical property
ability/inability of a substance to to change into other substance(s)
physical change
a change that alters a substance but not its composition

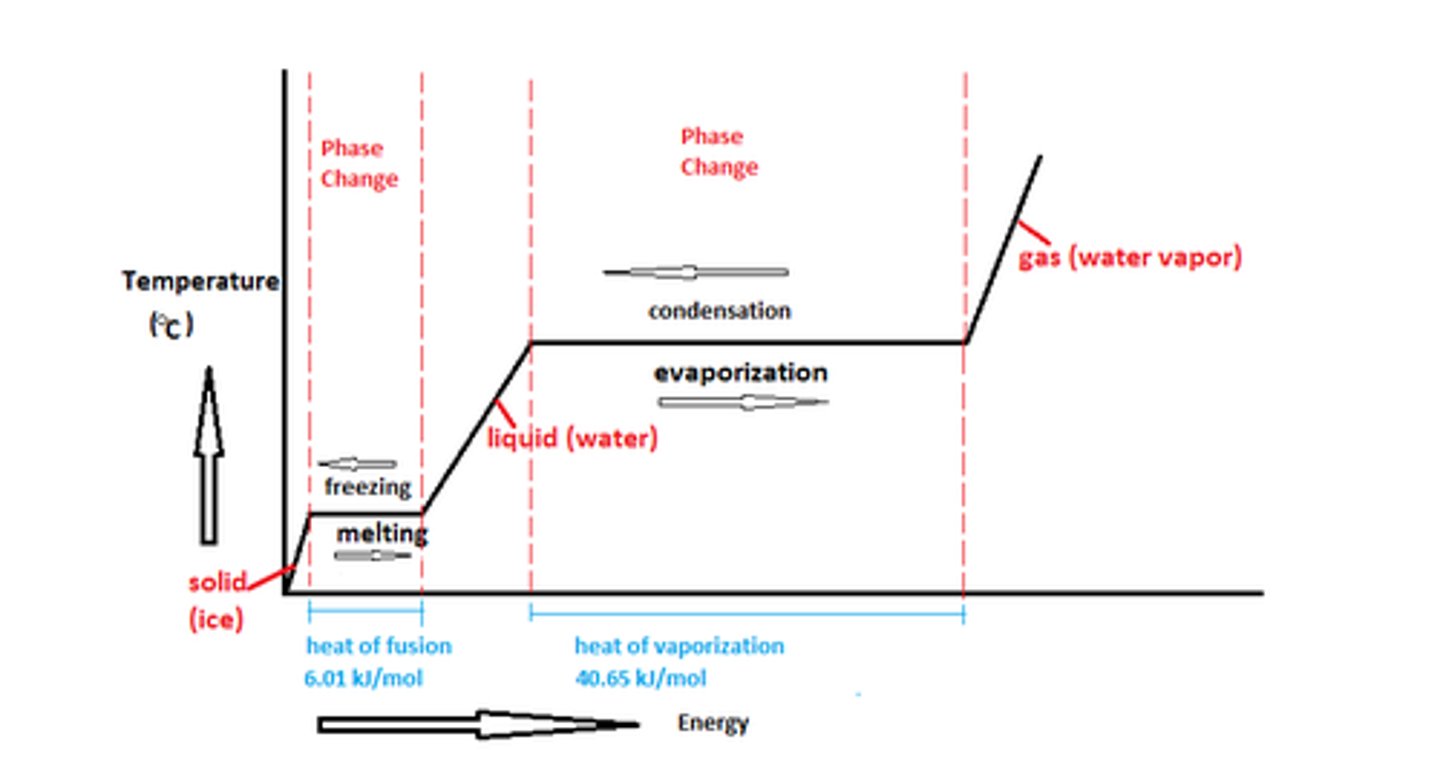
phase change
transition of matter from one state to another
chemical change
one or more substances changes into another substance
law of conservation of mass
mass is not created or destroyed during a chemical reaction
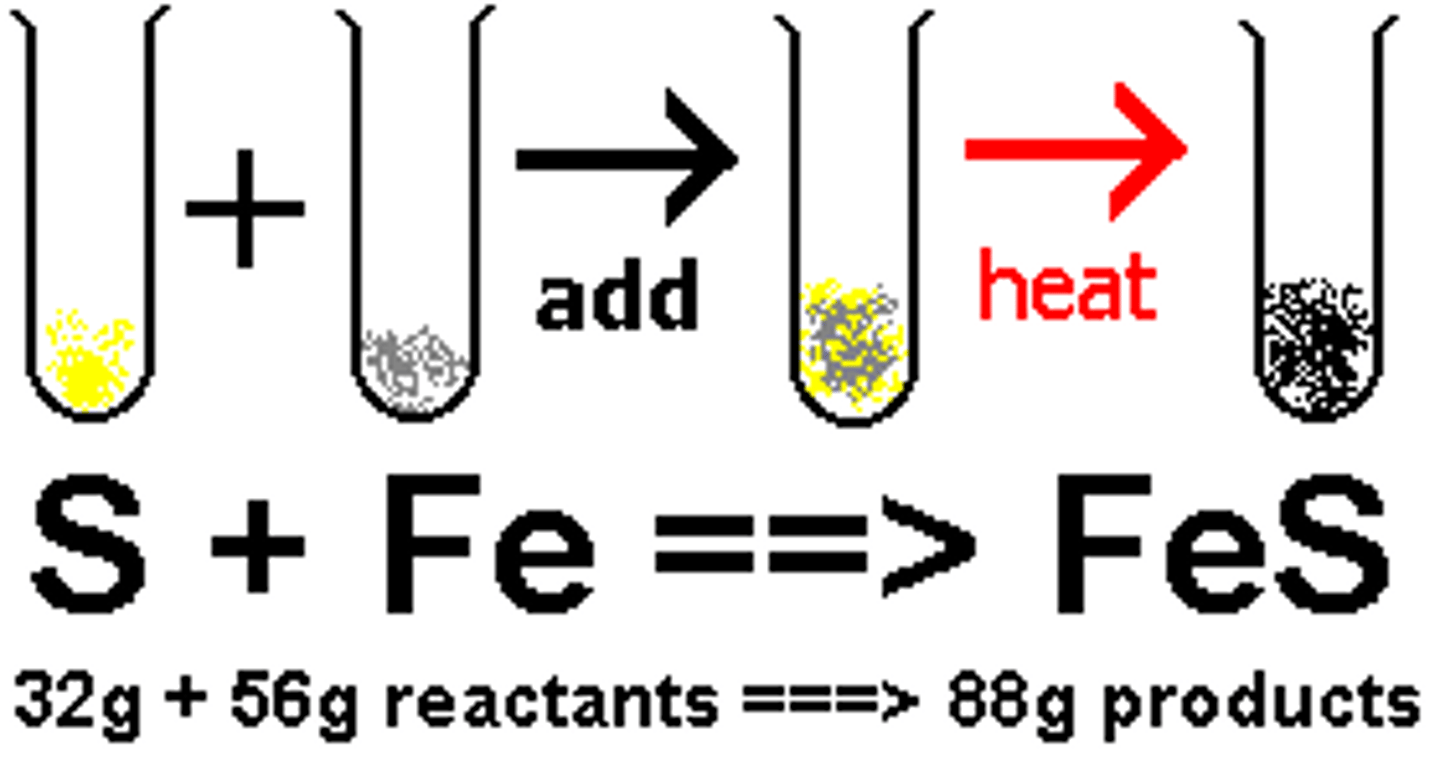
mixture
a combination of two or more pure substances, both retain its individual chemical properties

heterogenous mixture
mixture that doesn't blend smoothly, substances stay distinct

homogenous mixture
constant composition through out, always a single phase.

solution
another word for homogenous mixture

filtration
technique for separating a solid from a liquid.

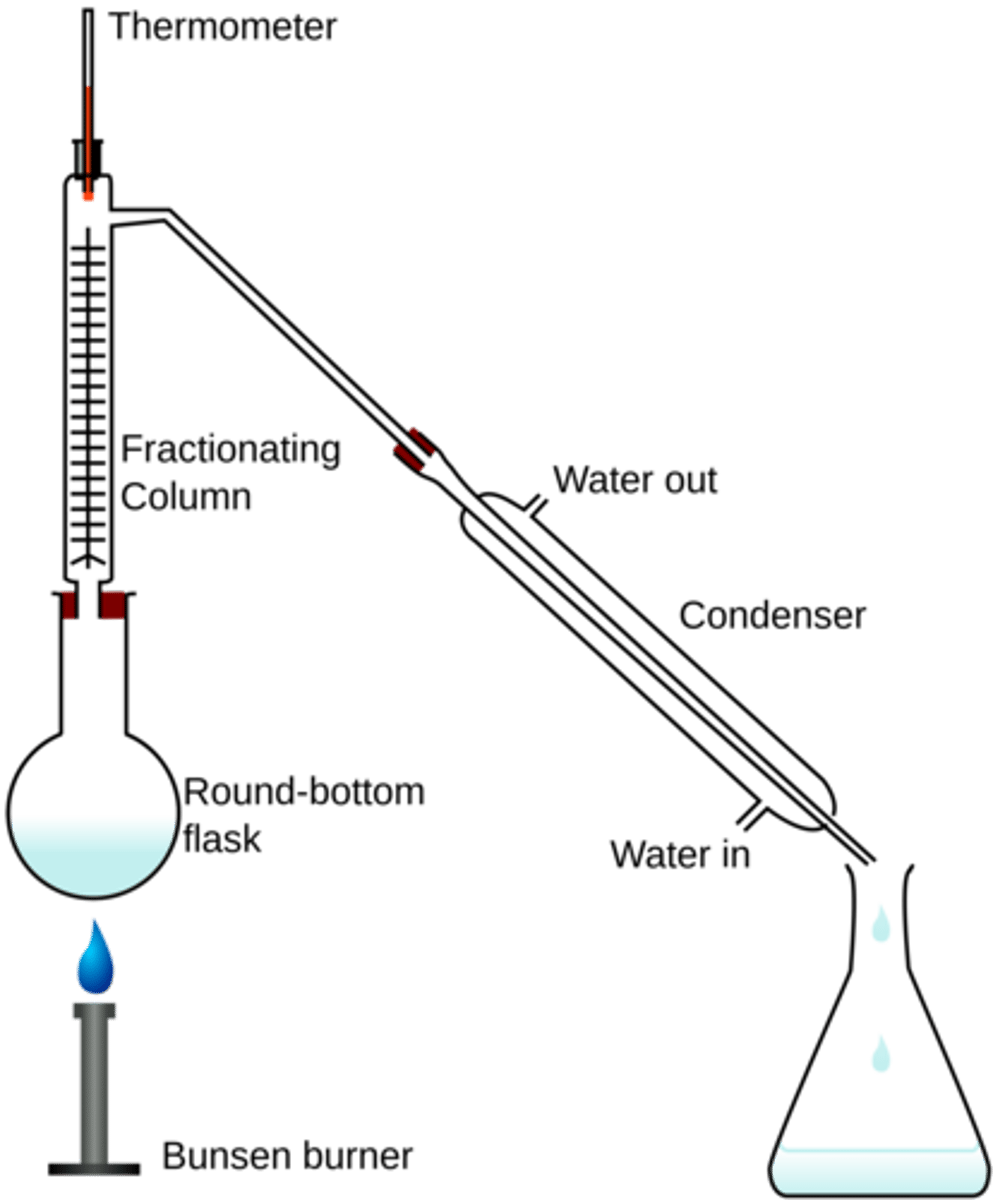
distillation
uses boiling to separate substances that have different boiling points
crystallization
separation technique, results in formation of pure solid particles of a substance from a solution containing the dissolved substance

sublimation
solid changes to a vapor without going through liquid phase

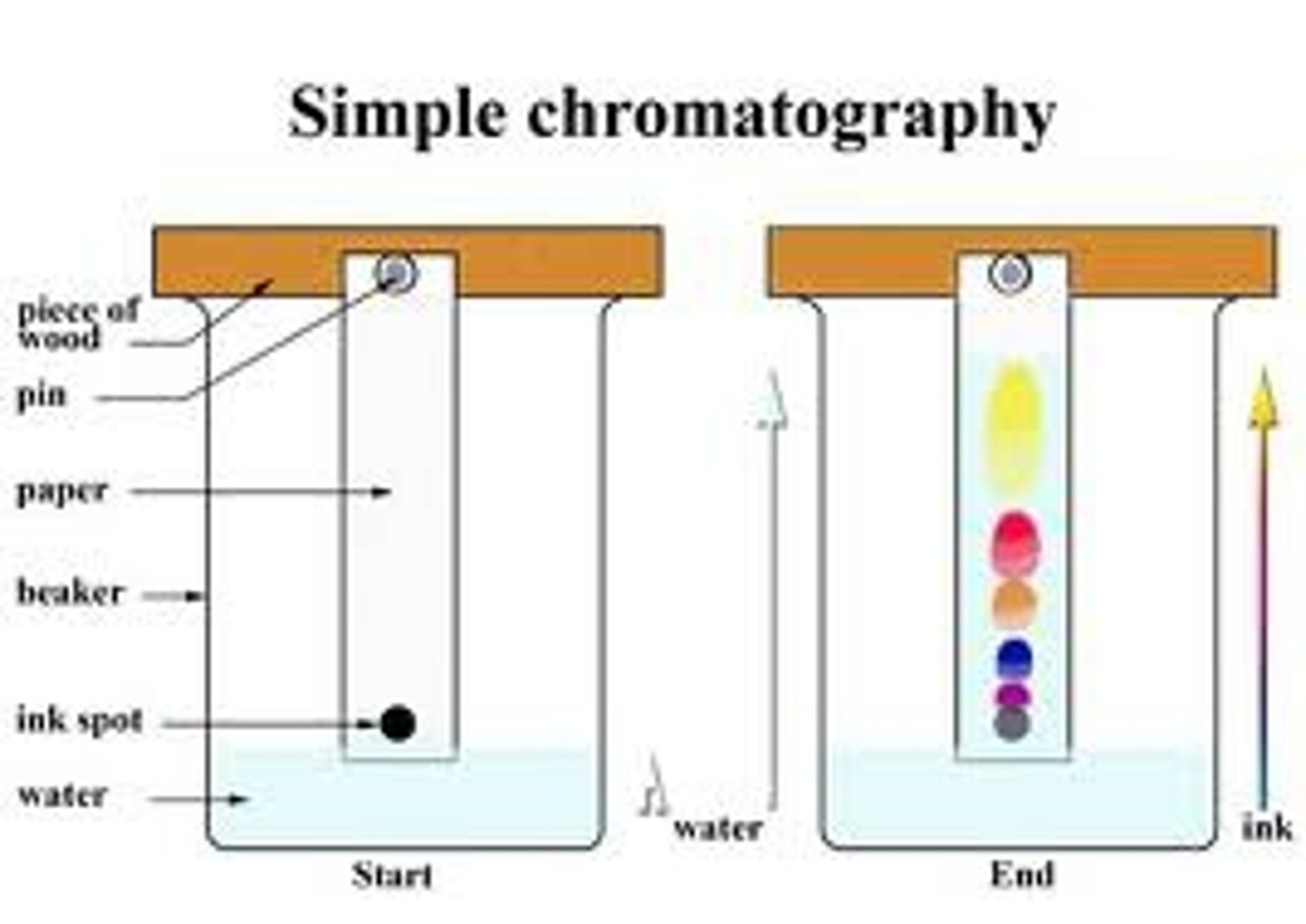
chromatography
technique that separates components of mixture dissolved in gas or liquid based on the ability of each component to travel across the surface of a fixed substrate
element
pure substance that can't be separated
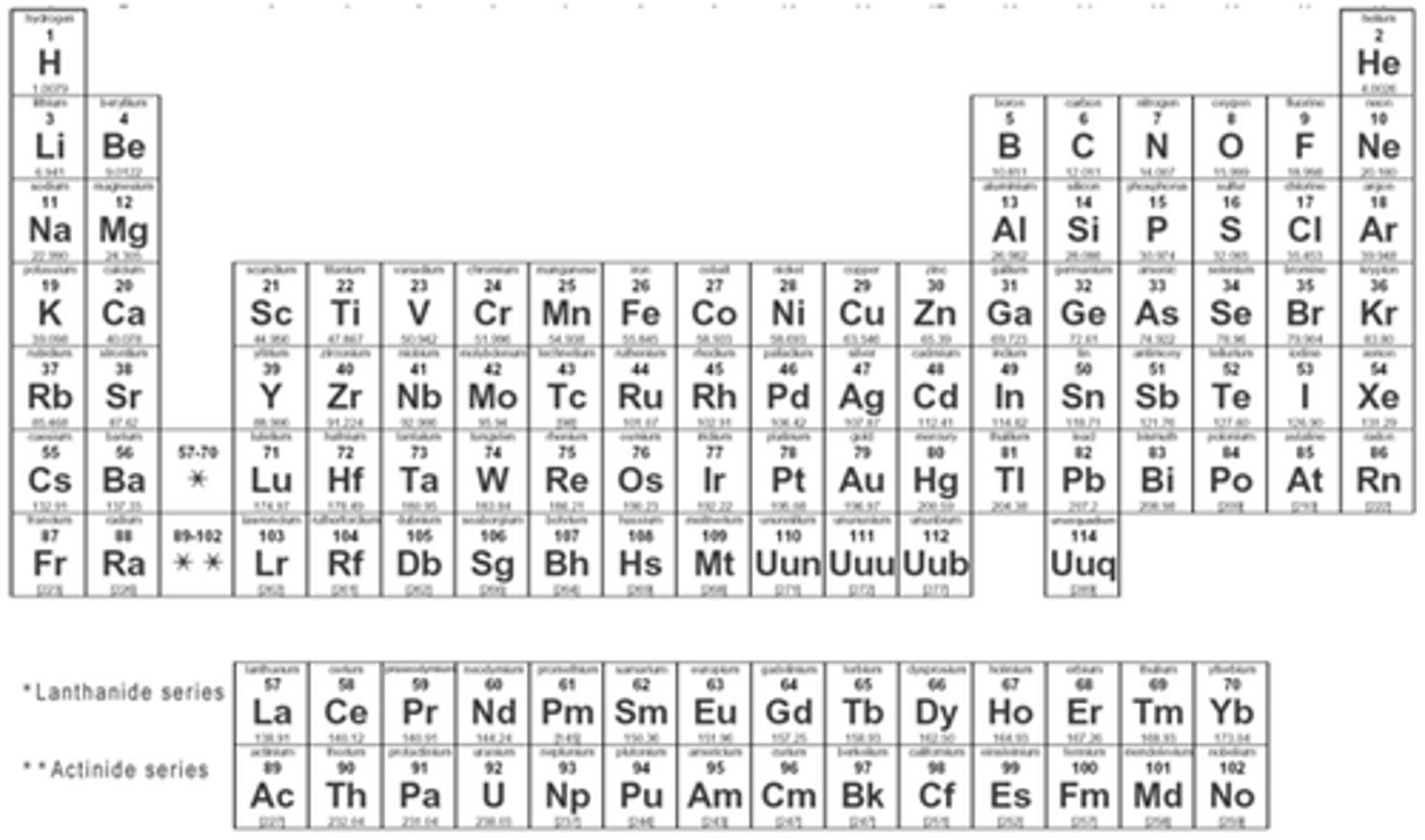
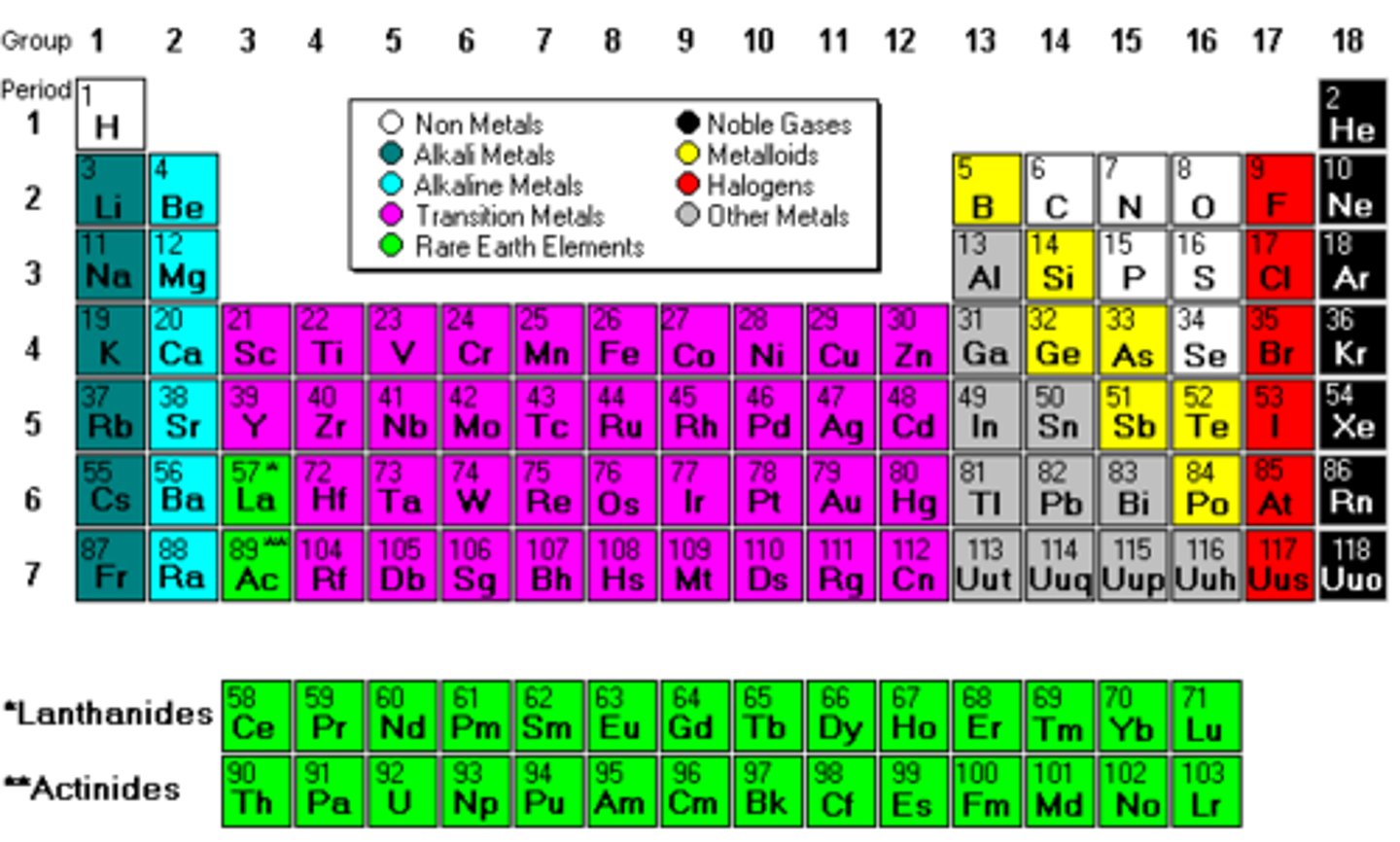
periodic table
organizes elements into a periods and families
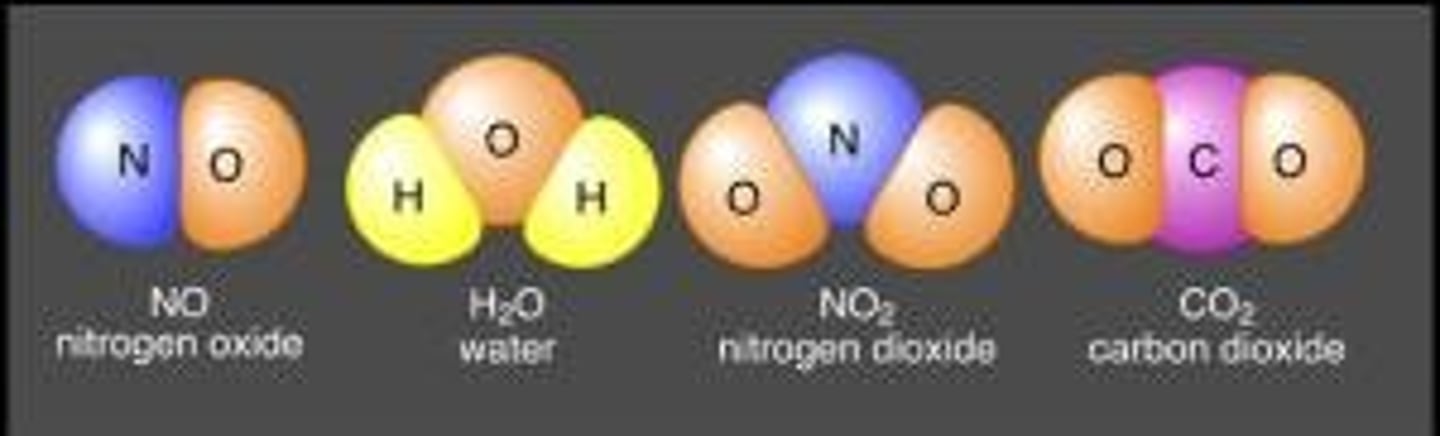
compound
made up of one or two different elements that are combined chemically
law of definite proportions
states that a compound is always composed of same elementsin same proportion by mass
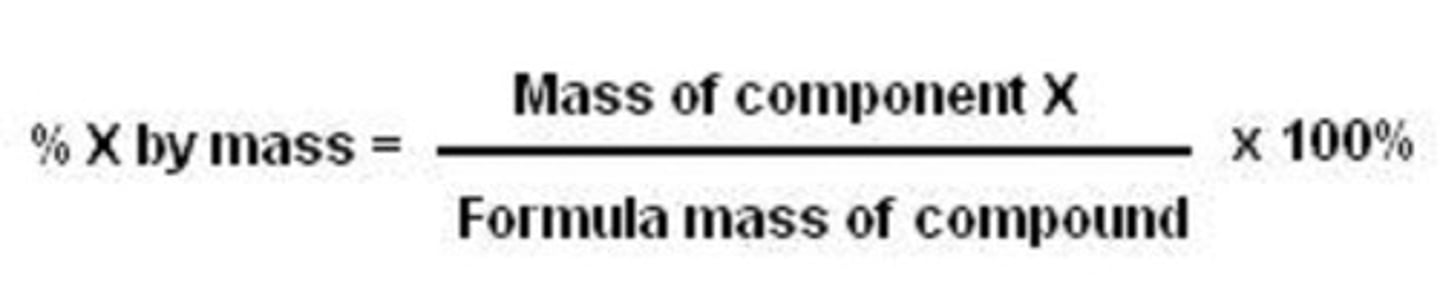
percent by mass
ratio of mass each element to the total mass of compound expressed as a percentage
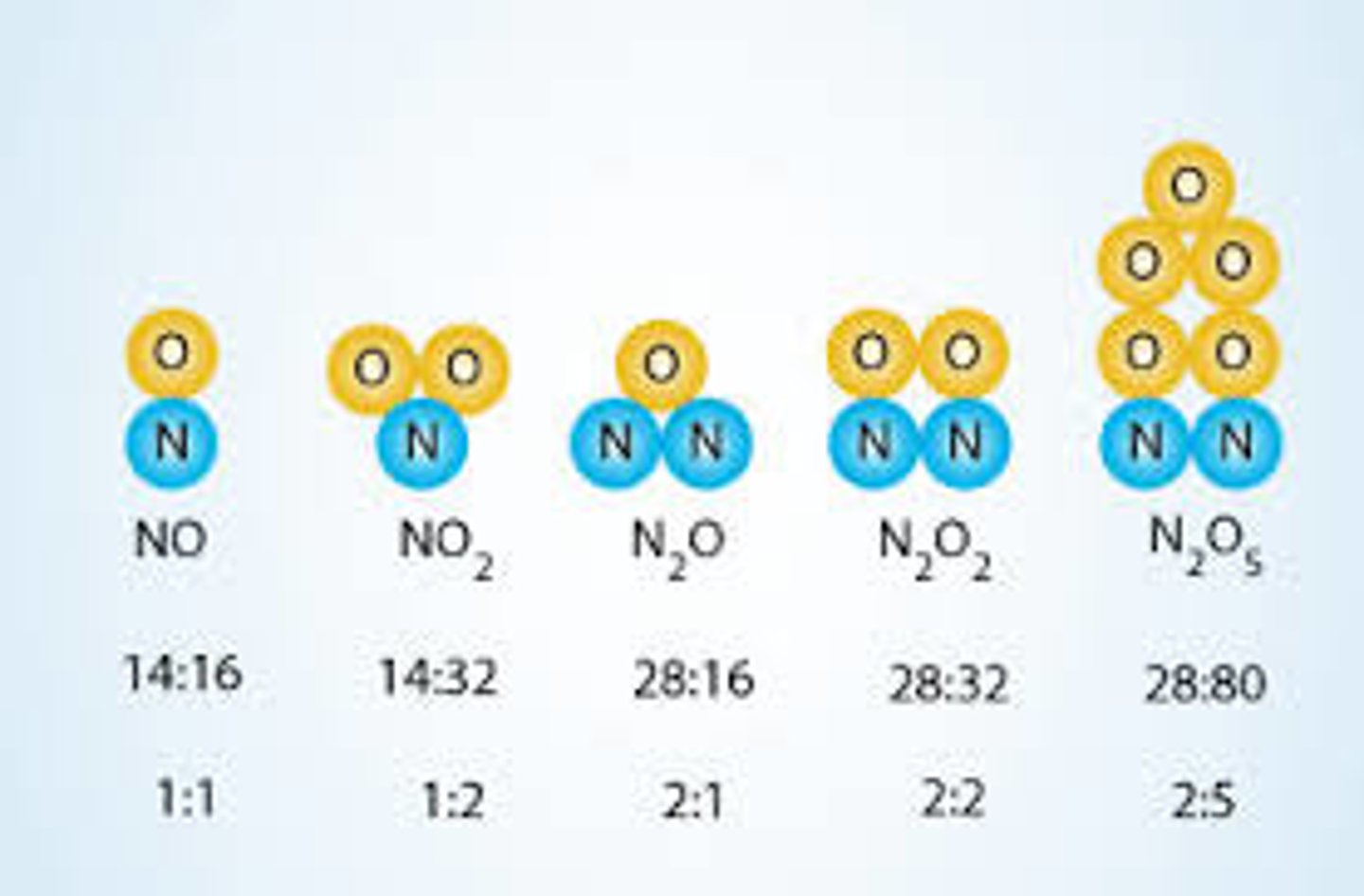
law of multiple proportions
says that when different compounds are formed by combination of same elements, different masses of one element combine with the same fixed mass of the other element in a ratio of small whole numbers