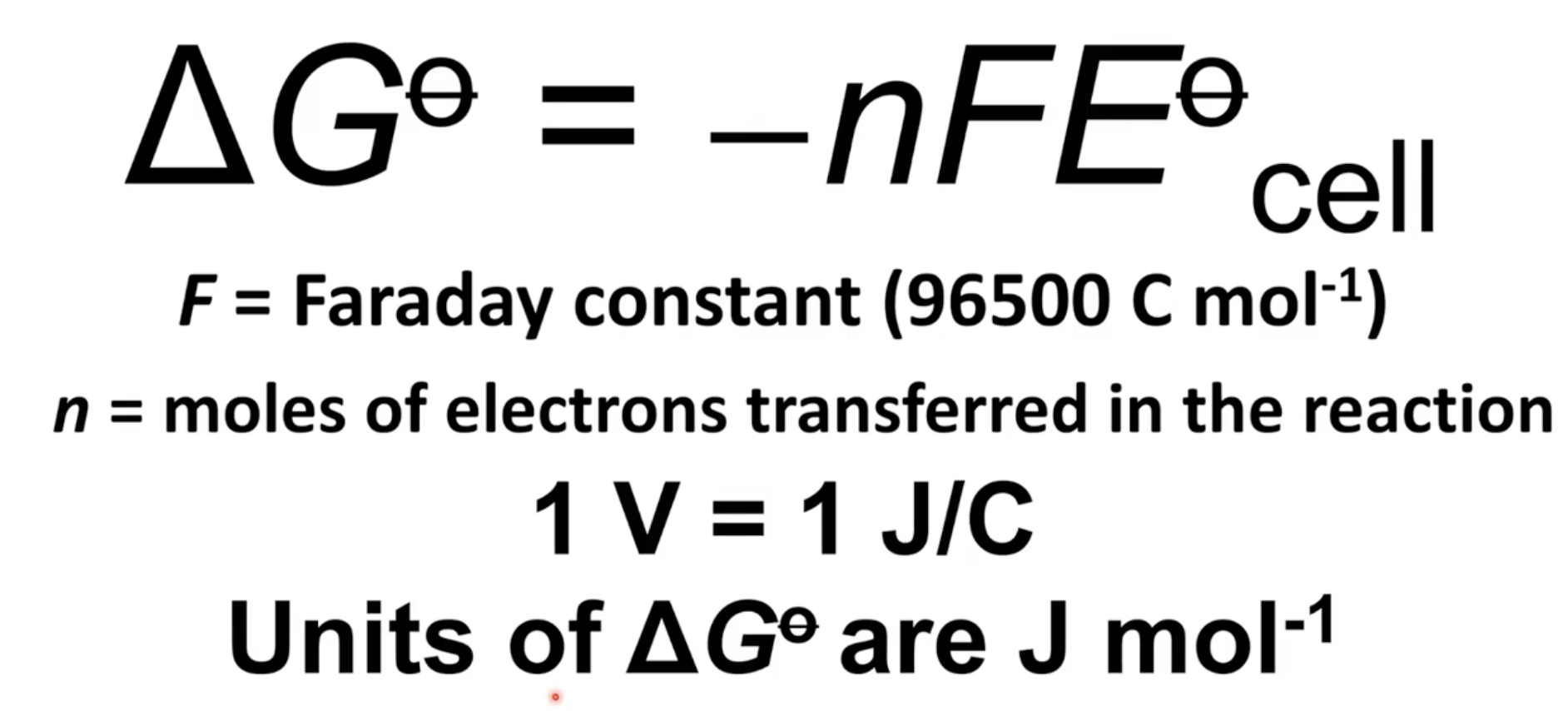R3.2.12, R3.2.13, R3.2.14 Standard electrode potentials & Cell potential
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
Standard electrode potential
Electrode potential of a half-cell measured under standard conditions relative to the SHE
SHE - Standard hydrogen electrode, assigned value of 0.00V

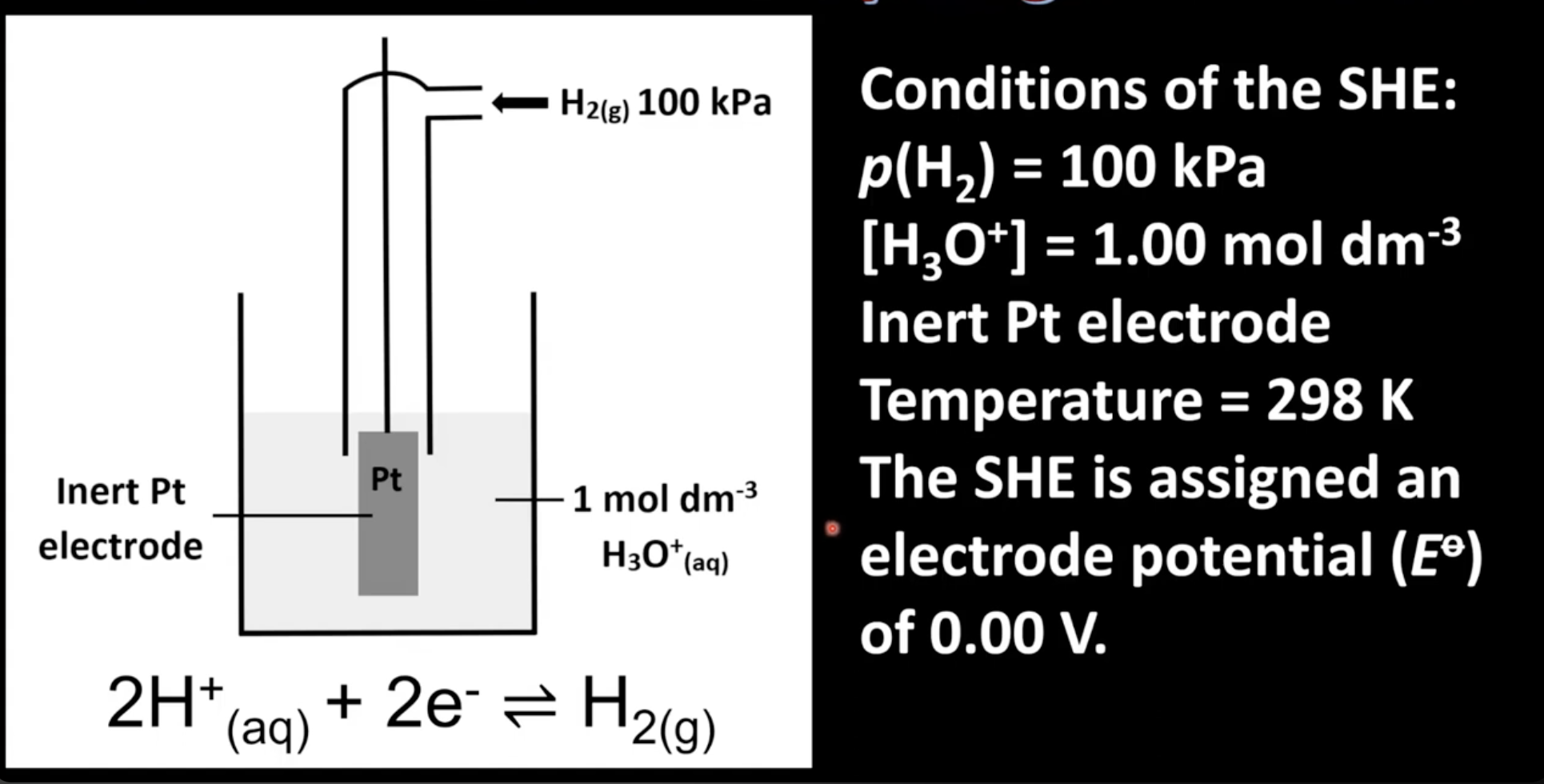
Standard hydrogen electrode conditions
Hydrogen pressure of 100 KPa
Solution of hydronium ions with a concentration of 1M
Inert platinum electrode
Temperature of 298K
Hydrogen either reduced to form hydrogen gas, or oxidized to form hydrogen ions
How SHE is used
SHE connected to half-cells to measure electrode potential
Reading of voltmeter tells us direction of electron flow
Positive voltage means electrons are flowing from half-cell to SHE
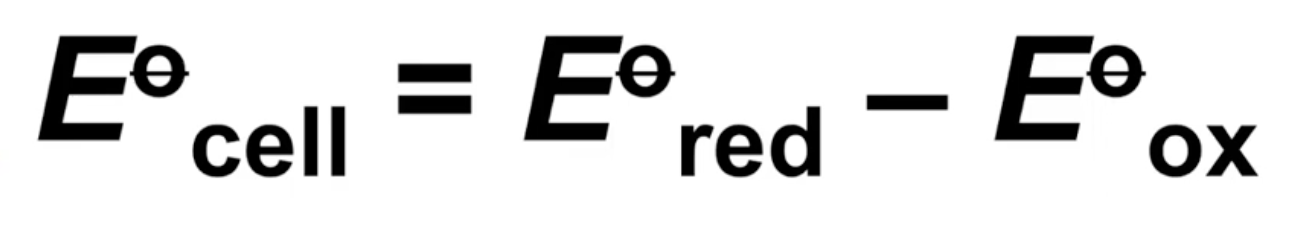
Sign of standard electrode potential
Positive means half-cell will undergo reduction when connected to SHE (will be cathode)
Negative means half-cell will undergo oxidation when connected to SHE (will be anode)
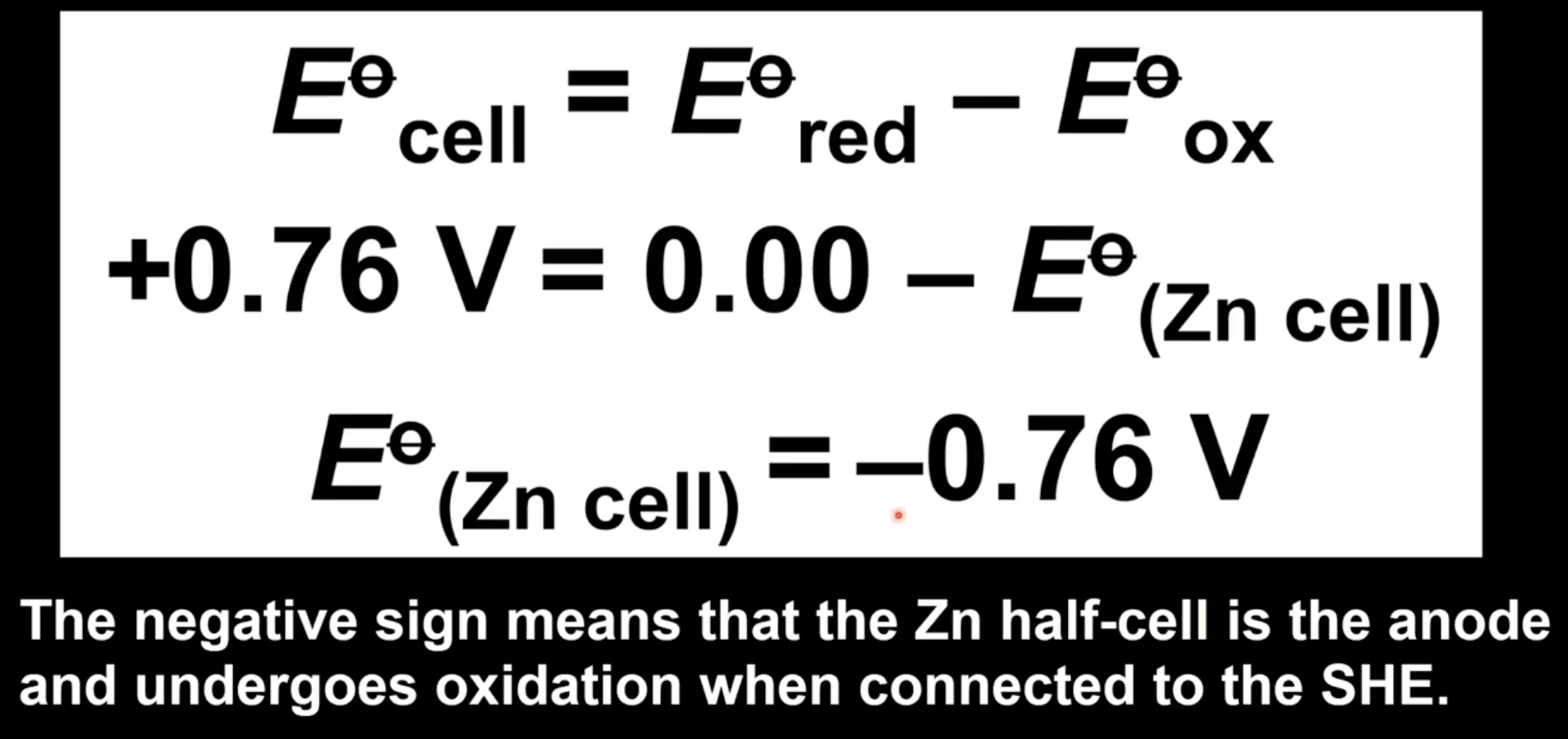
Table of standard electrode potential trends
More positive means reduction half-reaction occurs more readily
More negative means reduction half-reaction occurs less readily
More positive elements are better oxidizing agents
More negative elements are better reducing agents
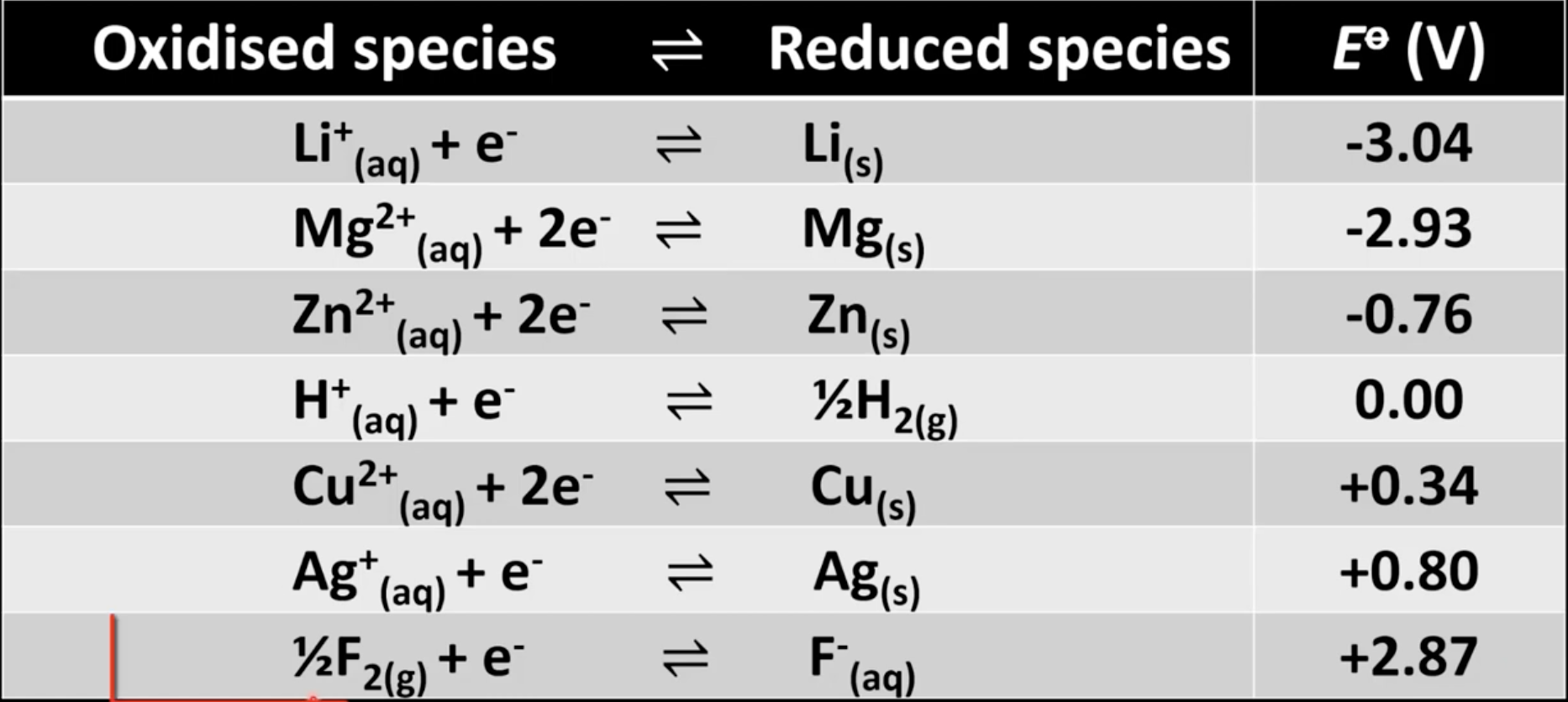
Which half-cell will be the anode and cathode?
Half-cell with more negative standard electrode potential is anode
Half-cell with more positive standard electrode potential is cathode
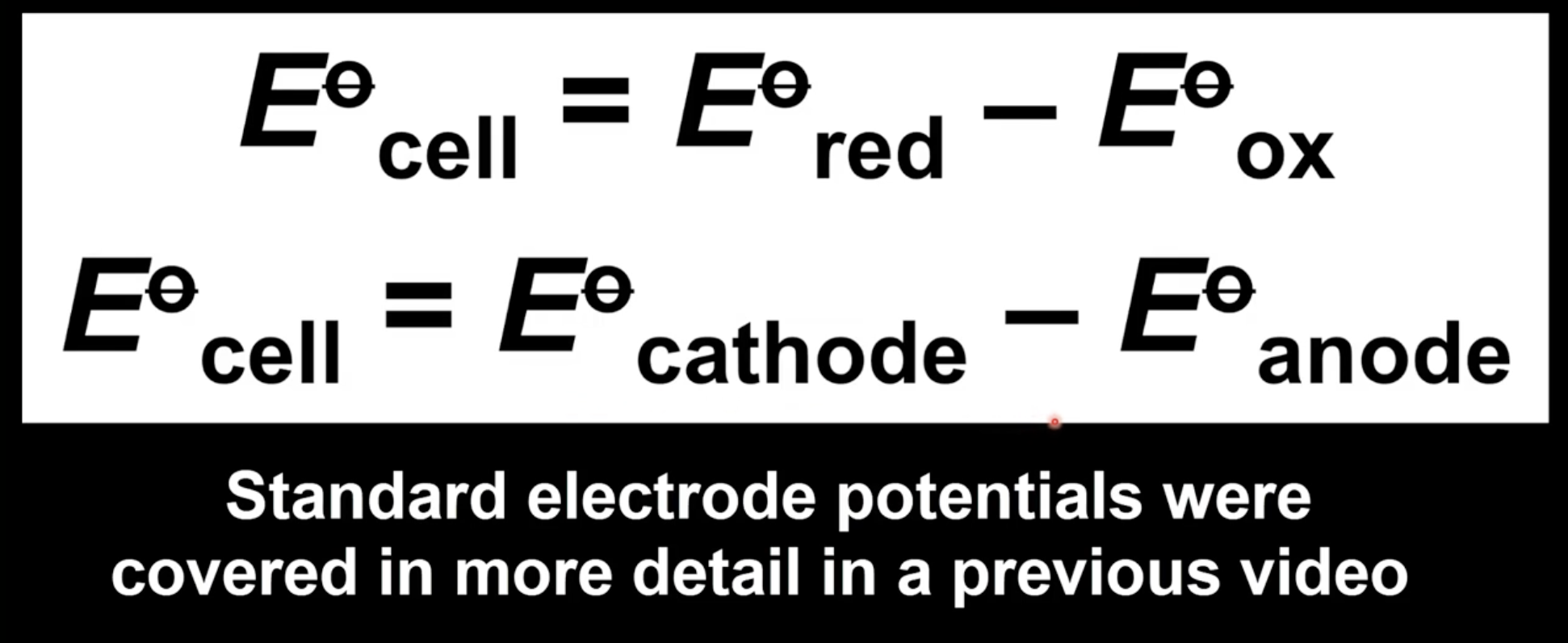
Calculating cell potential
Subtract standard electrode potential of half-cell undergoing oxidation from one undergoing reduction
Subtract standard electrode potential of anode from cathode
Relationship between cell potential and spontaneity
Positive cell potential means ΔG is negative (spontaneous)
Negative cell potential means ΔG is positive (non-spontaneous)
Zero cell potential means reaction is at equilibrium
Equation for calculating Gibbs free energy from electrode potentials
Units of Gibbs free energy are J/mol