rp4 - beetroot permeability
1/11
Earn XP
Description and Tags
named variable = alcohol conc
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
describe the method for making a simple dilution of 100% beetroot solution into 0, 20, 40, 60, 80 and 100% solutions and measurement of absorbance
use the formula m1v1 = m2v2 to calculate volume of 100% beet solution needed for set % conc
then make up the rest of the solution w/ distilled water
repeat for each % conc
label these standards 0, 2, 4, 6, 8 and 10
use a colorimeter to measure absorbance of each of the standards
plot calibration curve of extract against absorbance
describe the method for the second part of the experiment:
set up a water bath at 30 o C
with a second set of test tubes, add 2 cm³ of 100% alcohol to a test tube, put a bung in the tube and label the tube with the alcohol concentration.
repeat steps 2 and 3 with alcohol concentrations of 80%, 60%, 40% and 20%
put the tubes of alcohol in the water bath until temperature of the alcohol reaches 30 o C
blot 10 discs of beetroot with a paper towel to remove excess water
gently put two discs of beetroot in each of the five tubes and replace the bungs as soon as possible after doing so
leave the tubes in the water bath for 5 mins, shaking the tubes gently once every minute
remove the tubes from the water bath and immediately pour each solution into a clean test tube, being careful to label the tubes appropriately
throw the beetroot discs away
use calorimeter to measure the % absorbance of each beetroot solution and use your calibration curve to estimate the % conc of each solution.
record your results in a suitable table that includes colorimeter readings and your estimated % concs
how could we adjust the method to ensure that all of the beetroot tubes spent exactly the same time in the alcohol?
stagger incubation times
why was beetroot used?
beetroot cells contain high concs of betalin (a purple pigment) in their vacuoles
betalin cannot move across undamaged plasma membranes
by measuring the leakage of betalin, we can ∴ measure the degree of damage to cell membranes
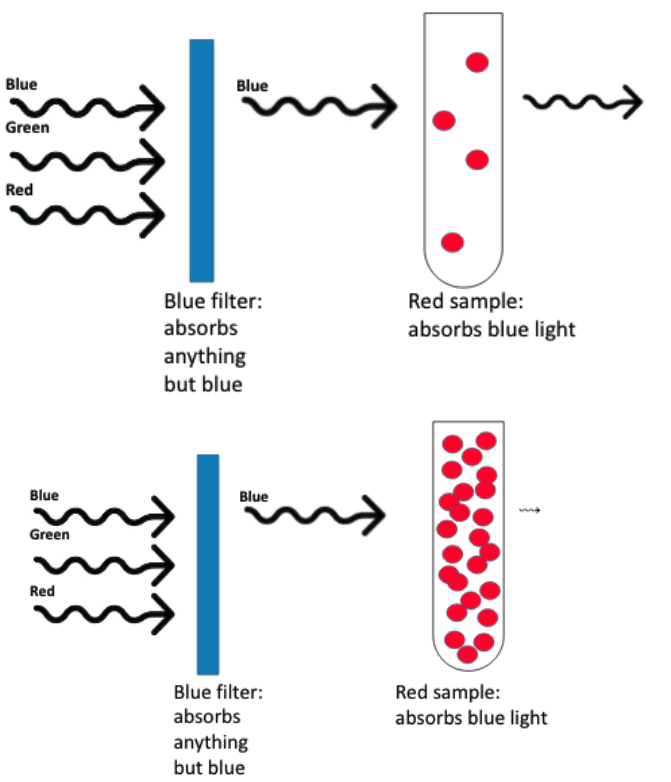
what is absorbance?
the amount of light absorbed by a solution
briefly explain how a calorimeter works:
at a low sample conc, little light will be absorbed → low absorbance
at a high sample conc, lots of light will be absorbed → high absorbance
give and explain any conclusions we may draw from this beetroot practical:
as the alcohol conc increases, the absorbance decreases, so the betalin leakage increases
this means that the cell membrane permeability increases
this is because the alcohol causes the cell membrane to rupture, releasing betalin from the cell
the higher the alcohol conc, the more disruption to the cell membrane and so more betalin is released

give one way in which the student could ensure the first three beetroot cylinders were kept at 25 o C throughout the experiment (1)
measure temp (thermometer/probe) at intervals and use appropriate corrective measure

give 2 variables the student did not control in her procedure (2)
length and diameter/SA/V/mass of cylinder/weight of cylinder (ignore shape/size)
time in solution (ignore ‘time’ if unqualified)
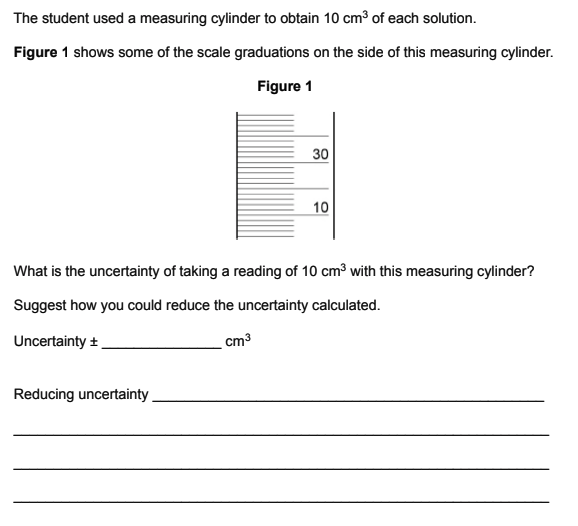
2/1 = 1 cm³
use instrument w/ closer/finer/smaller intervals/graduations/scale (ignore ‘higher resolution’)
how can we calculate uncertainty and % error?
uncertainty = no. of readings taken x smallest increment/2
% error = uncertainty/reading x 100
using the above diagram, what can you conclude about the damage caused to beetroot cells by water, ethanol, HCl and different temps? provide explanations for your conclusions (4)
water/25oC caused no damage/no pigment relase (in E)
damage to cell membrane
ethanol/HCl caused some/similar/identical damage OR 70oC caused most damage
by ethanol dissolving phospholipid bilayer/by HCl altering membrane protein
by 70oC denaturing/altering membrane protein OR increasing fluidity/permeability of membrane