Glycolysis
1/65
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
66 Terms
what makes ATP
consumption of glucose
is all of the energy from glucose stored from ATP
no; only about 980 kj/mol while glucose has 2840 kj/mol
how does glucose oxidation happen
proceeds in a stepwise manner via enzymatic redox rxns. Not spontaneous combustion allowing capture of free energy in NADH, FADH2 and ATP rather than heat.
how to ATP synthesized
reactive intermediates and through proton gradient
low energy phosphate compounds
there is an investment of ATP to make these compounds
G6P
F1,6BP
Glycerol 3- phosphate
cells form ATP via two ways
substrate level phosphorylation and oxidative phosphorylation.
substrate level phosphorylation
substrates are activated to form unstable compounds which can donate a phosphate group to ADP to form ATPphosphate ADP to form ATP
oxidative phosphorylation
electron carriers like NADH and FADH2 drive protons across the membrane, which is used to generate ATP via F1-ATP synthase (active transport run backward).
principles of enzymatic catalysis in glycolysis
Proximity: bringing partners together in correct orientation Protection of reactive intermediates Transition state stabilization Direct functional group catalysis: Acid/base, etc. Covalent enzyme intermediates: Like Ser protease Assistance from cofactors and metals Regulation through feedback mechanisms
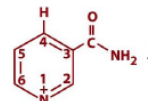
structure of NAD+
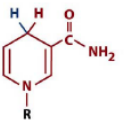
structure of NADH
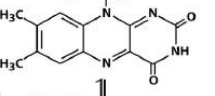
structure of FAD
structure of FADH2
how do cofactors help in glycolysis
they help progress glycolysis
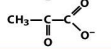
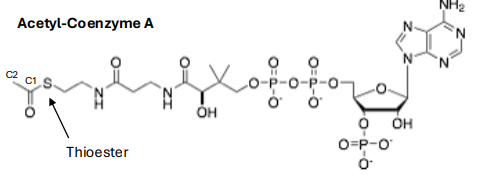
structure of acetyl co enzyme A
NAD+/FAD
used as electron sinks for carrying out oxidation reactions
Electrons are used to generate ATP
Acetyl CoA
activate carboxylates as thioesters for rxns at C1 and C2
why are thioesters more reactive
the C-S is less resonance stablized than a C-O bond making the carbonyl more electrophillic
preparatory
Step 1 to 5
payoff
Steps 6 to 10
irreversible steps in glycolysis
1, 3, 10
first step
Glucose to G6P
ATP to ADP
enzyme for first step
hexokinase
second step
G6P to F6P
enzyme for second step
phosphohexose isomerase
third step
F6P to F1,6BP
ATP—> ADP
enzyme for third step
phosphofructokinase-1 (PK1)
step 4
F1,6BP to Glyceraldehyde to Dihydroxyacetone phosphate (GAP and DHAP)
enzyme for step 4
aldolase
structure of glucose
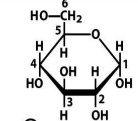
structure of G6P
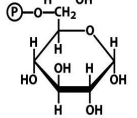
structure of F6P
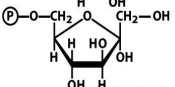
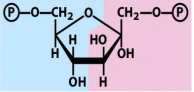
structure of f 1,6 bp
energy is consumed in what part of glycolysis
preparatory phase
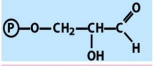
structure of GAP
structure of DHAP
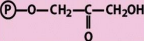
step 5
DHAP to GAP
enzyme for step 5
triose phosphate isomerase
after step 6 what must you do in order to get a balanced equation
multiply everything by 2
step 6
GAP (x2)—> 1,3 bisphosphoglycerate
2Pi—> 2H+
2NAD+ —> 2NAD+
H in GAP is replaced by phosphate from Pi causing release of H+. H from aldehyde goes to NAD+
enzyme for step 6
glyceraldehyde 3-phosphate dehydrogenase
step 7
1,3 BP glycerate—> 3 phosphoglycerate
2ADP—> 2ATP
enzyme for step 7
phosphoglycerate kinase
step 8
3 phosphoglycerate to 2 phosphoglycerate
enzyme for step 8
phosphoglycerate mutase
step 9
2 phosphoglycerate to phosphoenolpyruvate (PEP)
Release of water
enzyme for step 9
enolase
step 10
PEP to pyruvate
2ADP —> 2ATP
enzyme for step 10
pyruvate kinase
hexokinase
glucose C6 hydroxyl attacks ATP y phosphate forming G6P and ADP
irreversible process
reaction proceeds via an SN2 like phosphoryl transfer stabilized by Mg 2+ (pentavalent TS state)
PGI
interconverts G6P to F6P via a ring opening, proton transfer and base catalyzed ring closing sequence
Reversible
Active Glu residues alternately act as general acid and base; protonates the carbonyl oxygen; the other abstracts a proton from C2, forming an enediol intermate which will then tautomerize to the ketone.
(aldehyde—> ketone)
PFK
commits the sugar to glycolysis because F 1,6 biphosphate is not in other pathways; it must proceed to pyruvate
PFK catalyzes a second phosphorylation of F6P with ATP.
inhibit PFK
allosterically inhibit ATP
activate PFK
AMP
what is the purpose of the feedback mechanism of PFK
preventing unnecessary glycolysis
Aldolase
cleaves F 1,6 BP to GAP to DHAP via shiff base intermediate. (retro aldol rxn) with an active site composed of lys. The imine promotes the carbon to carbon cleavage
Reversible
TIM
cleaves DHAP into GAP
catalytically perfect because the reaction rate is limited only by substrate diffusion into the activate site
kcat/km= 10^-9 s^-1
Functions via acid-base catalysis and stabilization of enediol transition state. Prevents collapse to methylglyoxal by tight binding of enediol.
GAPDH
oxidizes GAP’s aldehyde to a carb acid level while simultaneously forming a high energy acyl phosphate
hosphorylates GAP to form phosphoanhydride from a reactive thioester interediate on Cys (similar to Ser protease).
NAD+ cofactor captures hydride (H- ) to form thioester, used later for energy
PGK
Phosphoanhydride bond is unstable, can be used to phosphorylate ADP. PGK formation of ATP, first direct energy forming reaction of glycolysis. PGK binds reactive substrates to orient them for reaction and prevent hydrolysis.
PGM
Phosphoglycerate mutase catalyzes isomerization of the phosphate group through covalent catalysis. Product phosphate comes from enzyme. PGM reaction is reversible.
enolase
Enolase generates a better phosphoryl donor for pyruvate kinase. Mg2+ ions are used to stabilize the doubly charged carboxyene intermediate. Lys and Glu sidechains carry out acid-base chemistry
PK
Final direct ATP-generating reaction in glycolysis. Tautomerization (2) to pyruvate drives reaction by preventing reverse step 1.
Allosterically activated by FBP for increased pyruvate at high [glucose]. Mg2+ stabilizes TS for ADP attack on PEP.
Regulated by Mg2+ ions; at high [ATP], Mg2+ is sequestered by ATP in other processes, so PK activity drops.
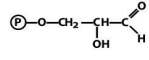
structure of glyceraldehyde 3 phosphate
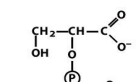
structure of 2 phosphoglycerate
structure of PEP
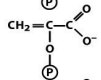
structure of pyruvate