proteins and enzymes
1/45
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
46 Terms
amino acids
the monomers from which proteins are made
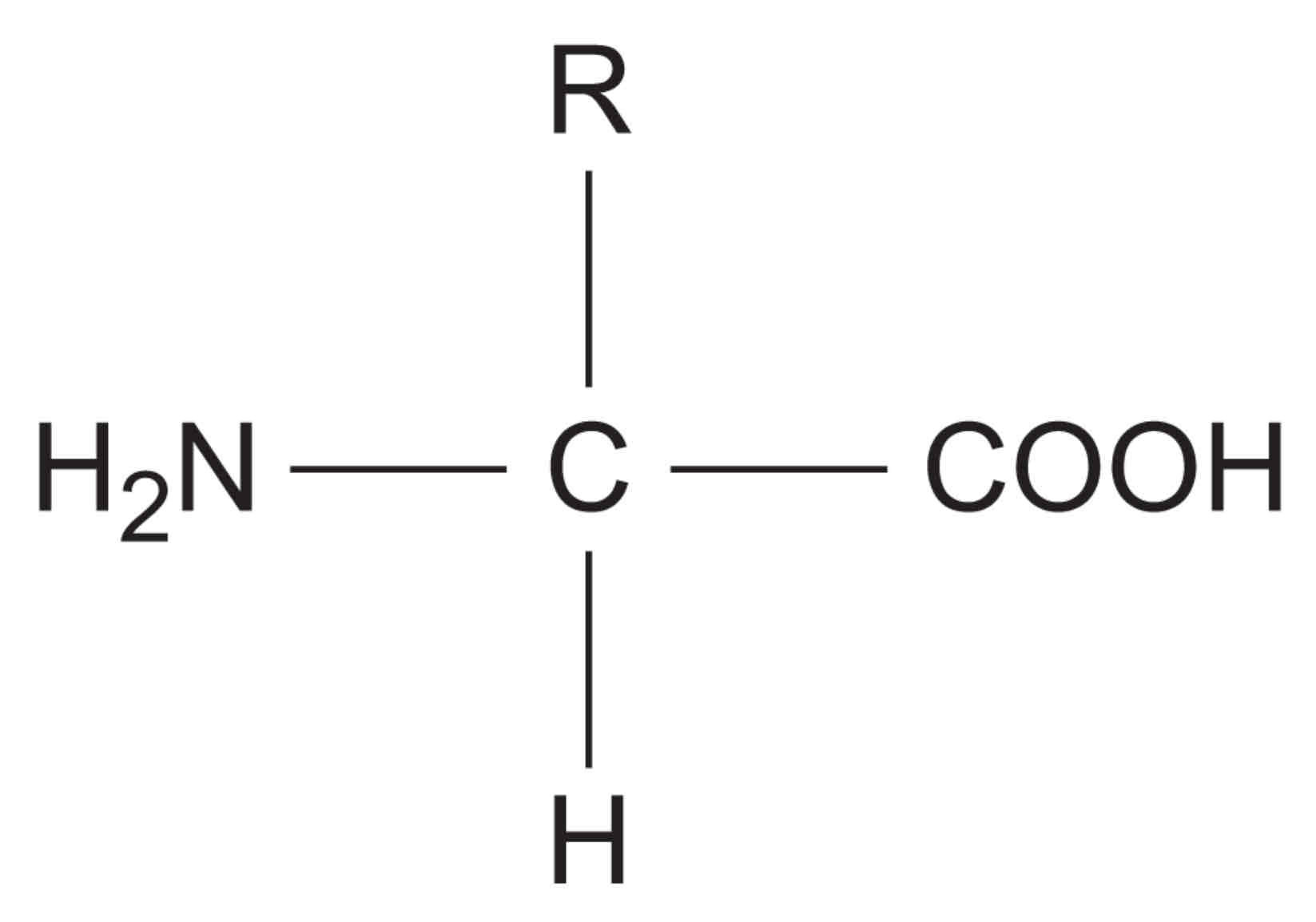
explain the general structure of an amino acid
NH2 represents an amine group, COOH represents a carboxyl group and R represents a carbon-containing side chain
how do the 20 amino acids that are common in all organisms differ?
only in their side group
how is a peptide bond formed?
by a condensation reaction between two amino acids
how are dipeptides formed?
by the condensation of two amino acids
how are polypeptides formed?
by the condensation of many amino acids
functional protein
may contain one or more polypeptides
proteins
proteins consumed are broken down into amino acids
amino acids are then transported to tissues in the body to create: muscle cells, antibodies, blood cells, hormones, etc.
what are the 4 levels of protein structure?
primary
secondary
tertiary
quaternary
what is the monomer of a protein?
amino acids
why are R groups important?
they can attract or repel each other
what causes polypeptides to fold?
R groups forming bonds with other amino acid R groups in the polypeptide
what bonds can R groups form?
disulfide bonds, hydrogen bonds, ionic bonds
where are hydrophilic R groups found?
on the outside of the protein
where are hydrophobic R groups found?
on the inside of the protein (away from water)
primary structure
polymerisation
sequence of amino acids → determined by DNA
determines proteins ultimate shape and therefore function
type of bonding: peptide
polymerisation
condensation reaction → amino acid monomers join together
secondary structure
hydrogen bonds cause folding of the polypeptide chain into alpha helix or beta pleated sheet
weak hydrogen bonds form
alpha helix
chain coils in a spiral shape
beta pleated sheet
chain runs parallel to itself
tertiary structure
further 3D folding of the polypeptide chain
type of bonding: disulfide bridges, ionic bonds, hydrogen bonds
disulfide bridges: strong bonds between S-S
ionic bonds: between amino acid side chains (weaker than disulfide bridges and easily broken by changes in pH)
hydrogen bonds: numerous but easily broken
if 1 amino acid in the primary structure is altered or changes position disulfide, ionic, and hydrogen bonds will form in different places → creates a different tertiary structure and different 3D shape
quaternary structure
more than one polypeptide chain bonded together
sometimes contains prosthetic groups (non-protein)
type of bonding: disulfide, ionic and hydrogen bonds
globular proteins
have complex tertiary and sometimes quaternary structures
folded into spherical (globular) shapes
usually soluble as hydrophobic side chains in centre of structure
roles in metabolic reactions
examples of globular proteins
enzymes, haemoglobin in blood
fibrous proteins
little or no tertiary structure
long parallel polypeptide chains
cross linkages at intervals forming long fibres or sheets
usually insoluble
many have structural roles
examples of fibrous proteins
keratin in hair and the outer layer of skin, collagen (a connective tissue)
biochemical test for proteins
biuret test (detects peptide bonds)
heat protein with biuret solution
positive result → biuret solution turns from blue to purple/lilac
negative result → biuret remains blue
enzymes
proteins
biological catalysts - reduce activation energy
facilitate chemical reactions - increase rates of reaction without being consumed
highly specific
how do enzymes react with their substrates?
both substrates must collide with sufficient energy to alter the arrangement of their atoms
an initial amount of energy is required to start the reaction off - activation energy
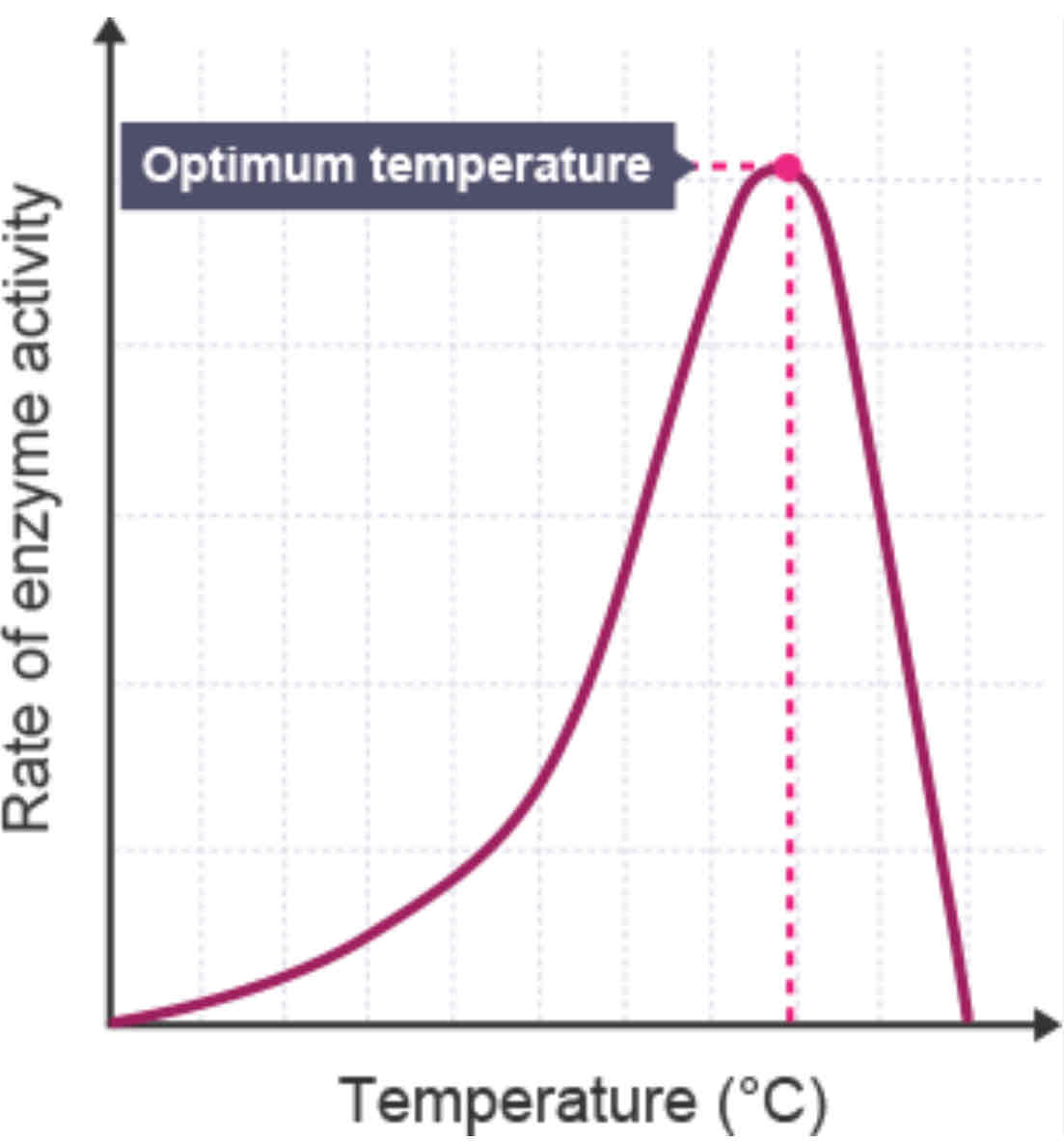
how does the temperature affect the rate of an enzyme-controlled reaction?
gives enzymes and substrate kinetic energy causing them to move around quicker resulting in more collisions → more e-s complexes form
optimum e-s complexes made at optimum temp (also optimum rate of reaction)
when temp exceeds optimum it denatures enzymes active site → bonds that hold enzymes 3D shape (H + I bonds) break → tertiary structure changes → active site no longer complementary to substrate → no more e-s complexes form
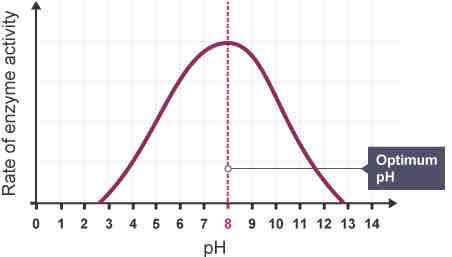
how does the pH affect the rate of an enzyme-controlled reaction?
enzymes have an optimum pH at which they work fastest (normally pH 7-8)
a few enzymes can work at extreme pH
in highly acidic or alkaline environments H+ or OH- ions affect ionic and hydrogen bonds, altering tertiary structure and shape of the active site
when pH exceeds optimum it denatures enzymes active site resulting in no more e-s complexes forming
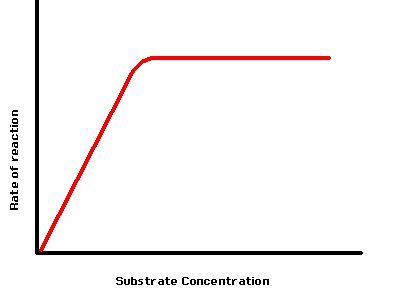
how does the substrate concentration affect the rate of an enzyme-controlled reaction?
higher substrate concentration increase rate of reaction as not all active sites are occupied
max rate of reaction at saturation point → all active sites are occupied
despite high conc of substrate rate of reaction would not increase any further as all the active sites are occupied
graph plateaus
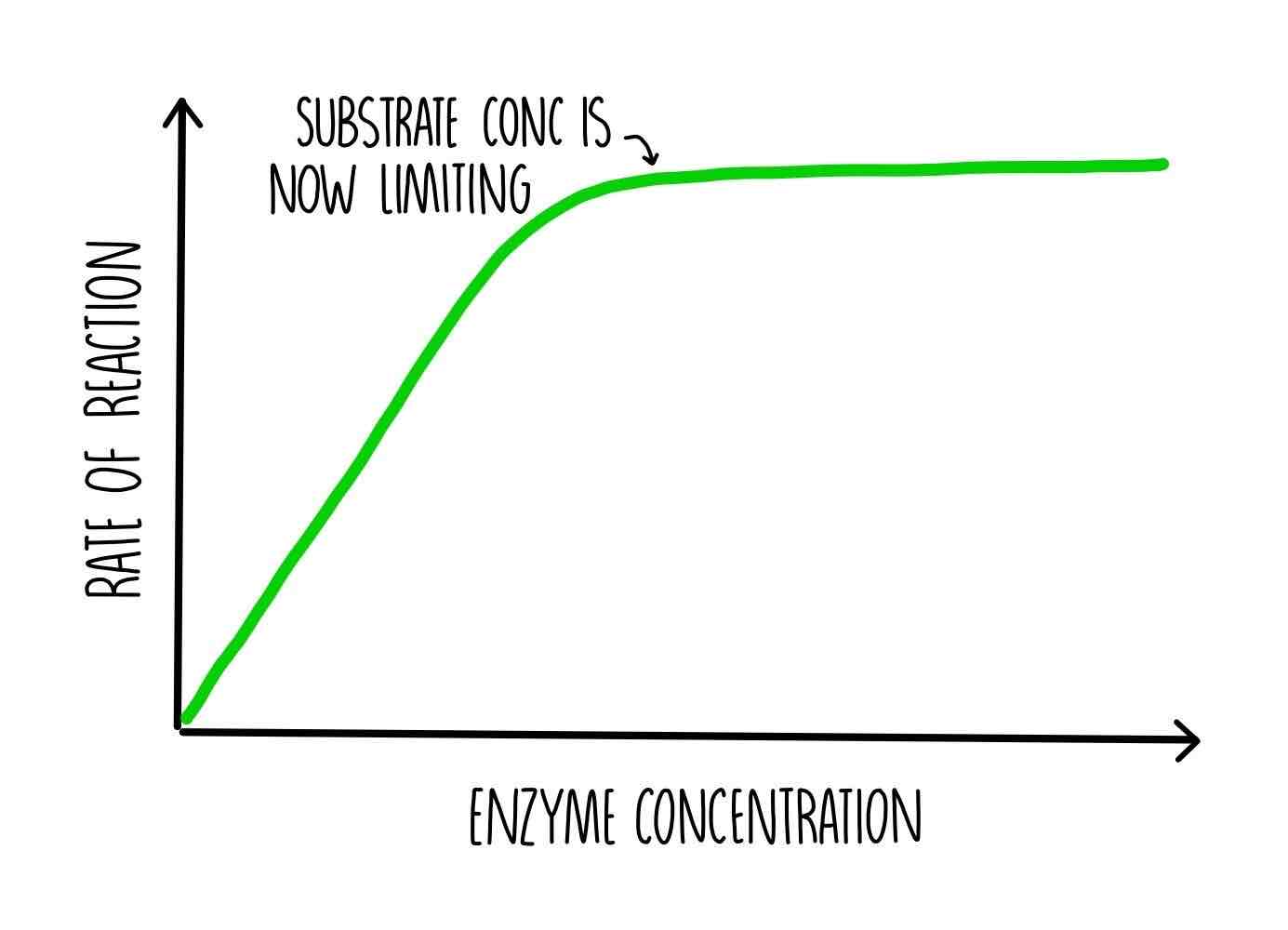
how does the enzyme concentration affect the rate of an enzyme-controlled reaction
substrate in excess; the rate of reaction increases as enzymes are re-used and the substrate continues to be available
substrate limited; the rate of reaction increases until the enzymes are limited by the concentration of substrate, no more substrate is available
lock and key theory
suggests enzymes and substrate are rigid structures
random movement, collision
substrate bind to enzyme’s active site, charged groups attract, distorting the substrate
forming e-s complex
products released, enzyme left unchanged, ready to bind again
strength of lock and key model
explains how the enzyme is specific; 1 substrate, will fit into active site
limitations of lock and key model
states the enzyme is rigid but enzyme structure is flexible not rigid
doesn’t explain how other molecules (e.g. activators and inhibitors) can bind to enzyme at sites other than active site and change its activity
the active site
the functional site of the enzyme
a relatively small number of amino acids
induced fit model
an enzymes shape is flexible
initially the substrate and active site of th enzyme are not complementary
the proximity of the substrate causes a change in the environment of the enzyme
leads to a change in the shape of the active site, this puts a strain on the substrate and distorts bonds, allows an e-s complex to form
why is the induced fit model a better explanation of enzyme controlled reactions than the lock and key model?
explains how other molecules effect enzyme activity (enzyme flexible) e.g. activators and inhibitors
explains how the activation energy is lowered (by putting a strain on the bonds)
factors that affect the rate of enzyme catalysed reactions
temperature
pH
substrate concentration
enzyme concentration
concentration of competitive inhibitors
concentration of non-competitive inhibitors
inhibitors
substances that directly or indirectly interfere with the functioning of the active site of an enzyme
what are the two types of inhibitors?
competitive inhibitors
non-competitive inhibitors
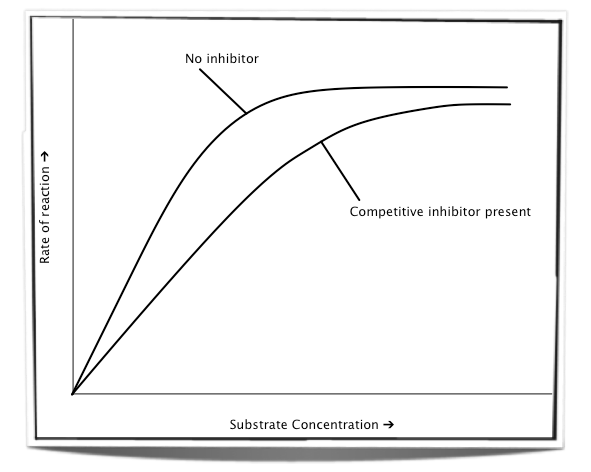
competitive inhibitors
similar molecular shape to substrate
bind to active site (not permanently)
this prevents the substrate from binding with the enzyme
increasing substrate concentration increases rate of reaction
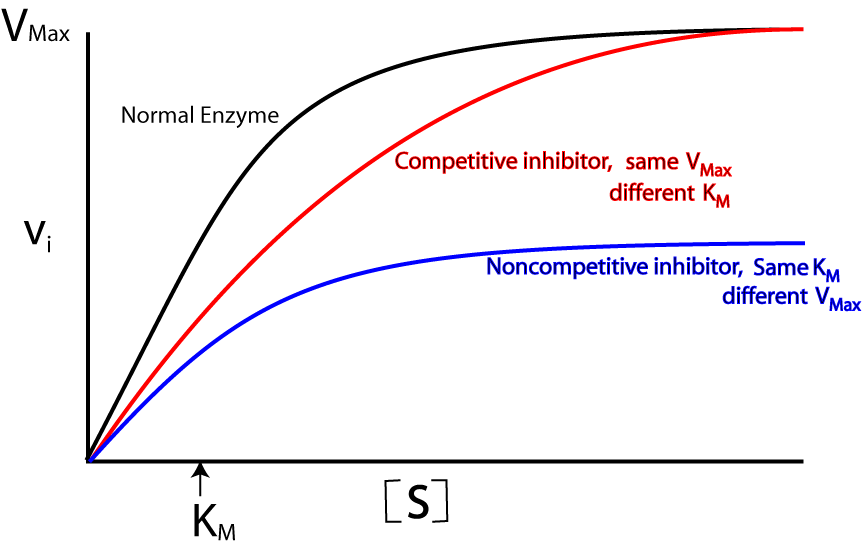
non-competitive inhibitors
bind to allosteric site
this alters the shape of the enzyme and its active site
the substrate can no longer bind to the enzyme → decreases rate if reaction
increasing substrate concentration will have no effect on rate of reaction
activation energy
the minimum amount of energy needed to activate the reaction
forming new bonds and breaking bonds requires energy
the specificity of an enzyme
tertiary structure
active site shape almost complementary to shape of substrate
substrate binds to active site
e-s complex is produced